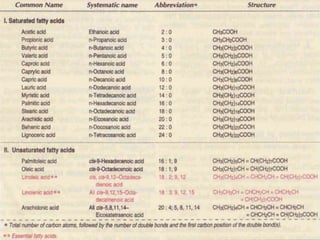
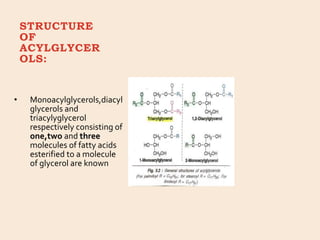
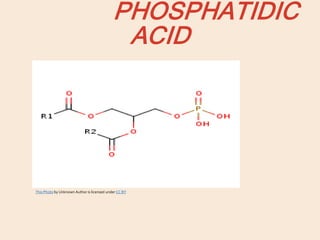
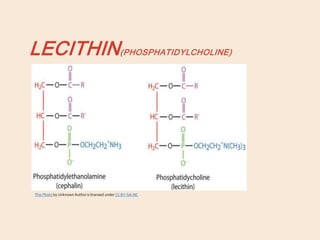
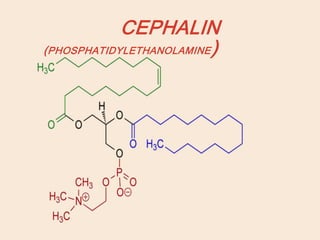
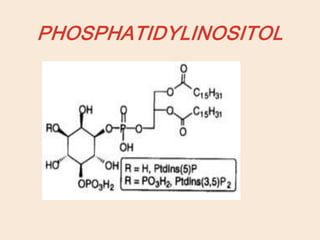
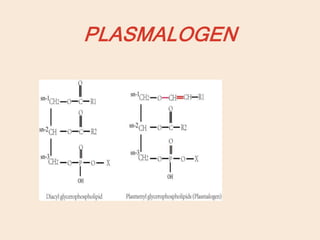
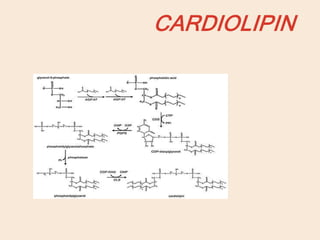
Lipids are organic substances classified into simple lipids, complex lipids, and derived lipids, with functions including energy storage, digestion facilitation, and structural support for cell membranes. Simple lipids consist of fats and oils, while complex lipids include phospholipids, glycolipids, and lipoproteins, essential for various biological functions. Fatty acids, critical components of lipids, are categorized as saturated or unsaturated based on their chemical bonds and play significant roles in human health and metabolism.