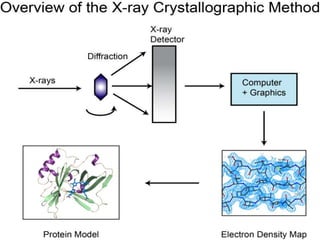
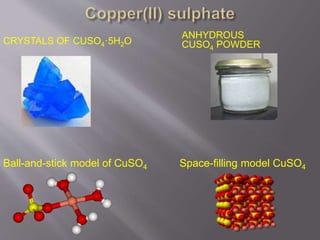
Crystallography examines the arrangement of atoms in solids. X-ray crystallography uses X-rays to determine the structure of large biomolecules like proteins by diffracting X-rays off of crystals and analyzing the diffraction patterns. Sodium chloride has an ionic crystal structure where sodium and chloride ions are arranged in a cubic close-packed pattern. Copper sulfate forms blue pentahydrate crystals and can be used as a fungicide or in organic synthesis and school experiments.