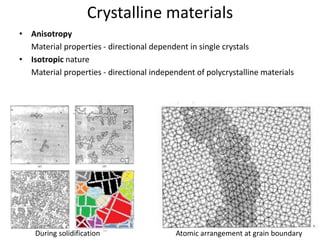
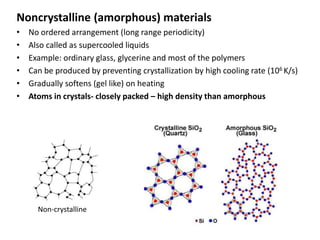
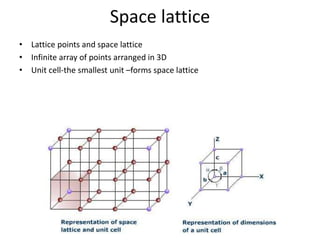
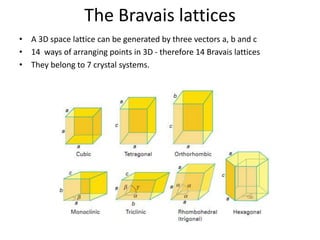
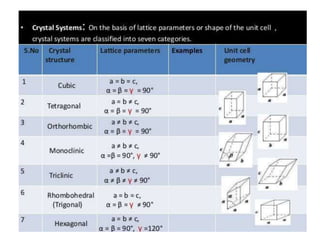
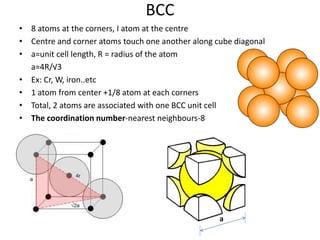
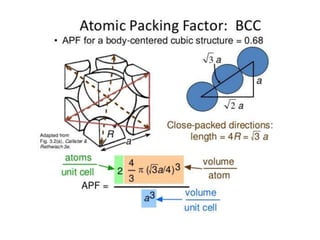
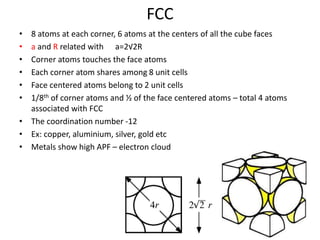
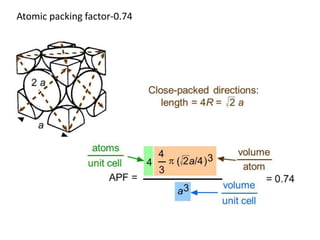
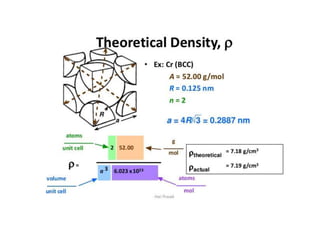
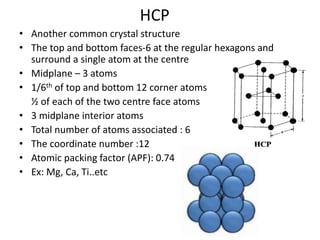
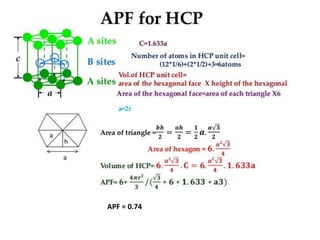
The document discusses the two principal forms of solids: crystalline and non-crystalline (amorphous) materials, detailing their structural differences, properties, and examples. It explains concepts such as space lattice, unit cells, and various crystal structures including body-centered cubic (bcc), face-centered cubic (fcc), and hexagonal close-packed (hcp). Additionally, it covers atomic packing factors and theoretical density calculations based on crystal structures.