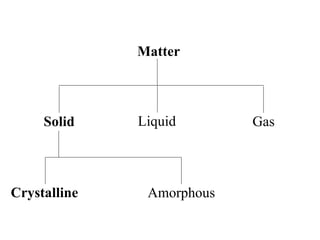
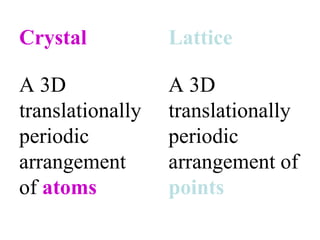
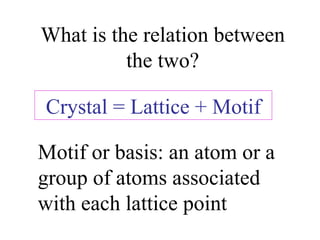
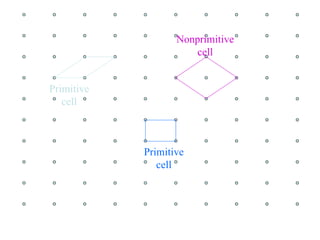
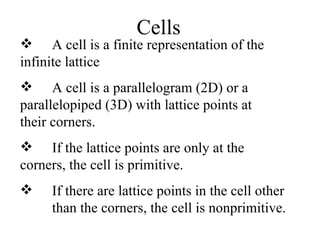
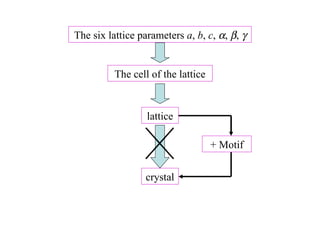
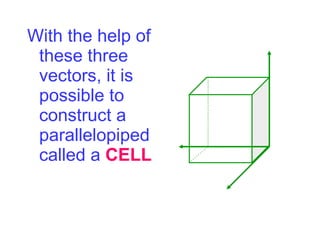
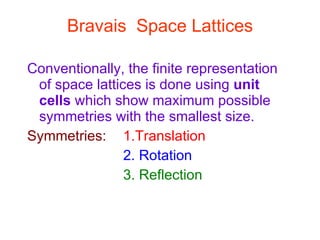
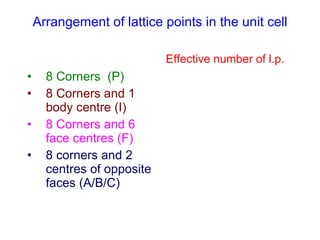
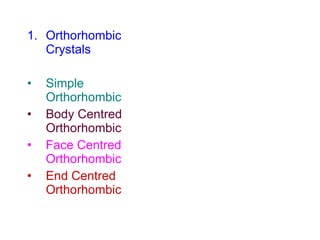
Crystals have basic building blocks called unit cells that are arranged in a periodic pattern described by a lattice. The unit cells contain motif groups of atoms associated with each lattice point. There are 14 possible types of space lattices belonging to 7 crystal systems characterized by distinct unit cell shapes and symmetries. The crystal structure is defined by the space lattice and the motif or basis group of atoms.