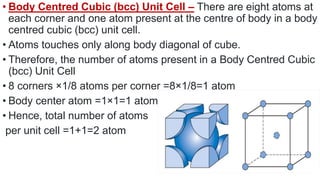
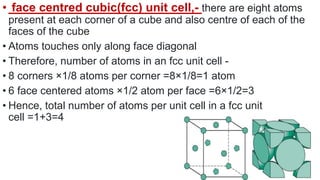
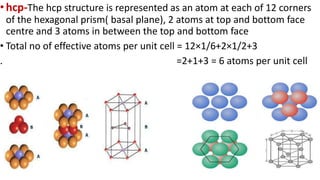
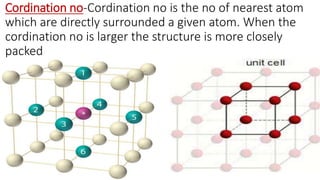
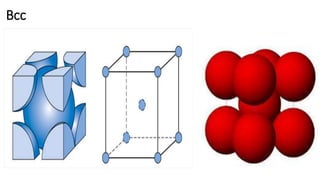
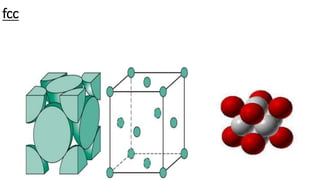
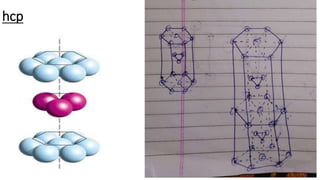
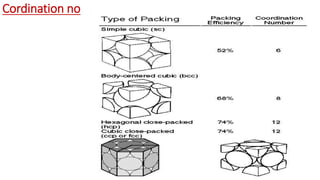
The document discusses the crystal structures of metals. The three main crystal structures are body-centered cubic (BCC), face-centered cubic (FCC), and hexagonal close-packed (HCP). BCC contains 2 atoms per unit cell with atoms at the corners and center. FCC contains 4 atoms with atoms at the corners and centers of the faces. HCP contains 6 atoms with atoms at the corners of the hexagonal prism and centers of the top and bottom faces.