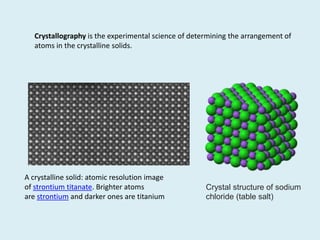
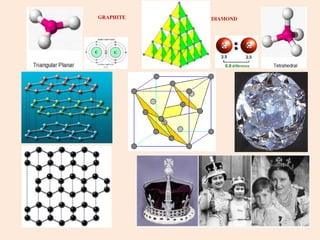
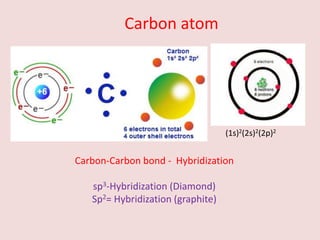
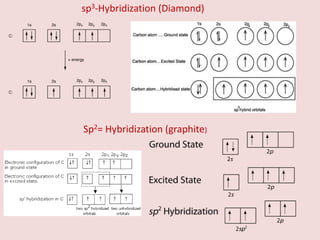
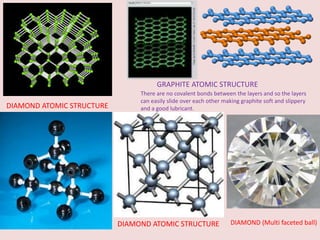
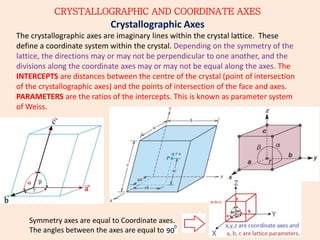
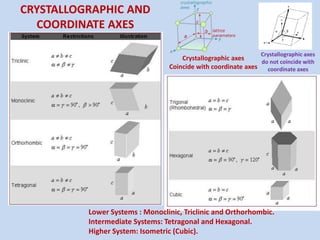
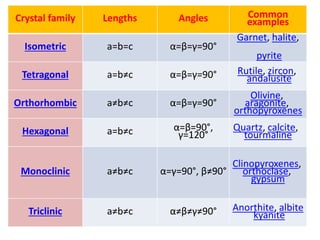
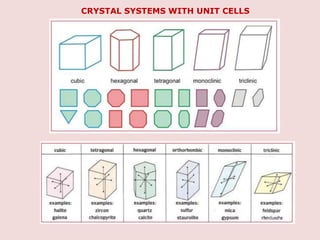
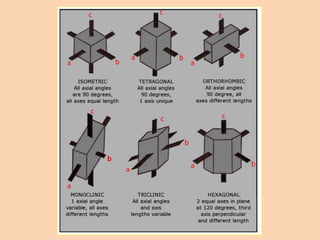
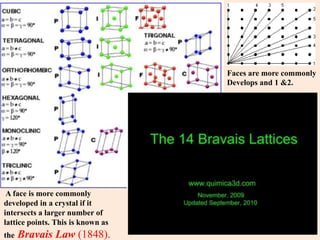
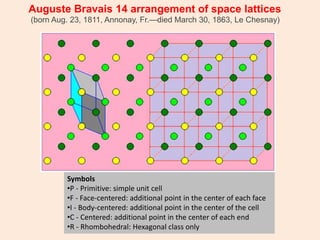
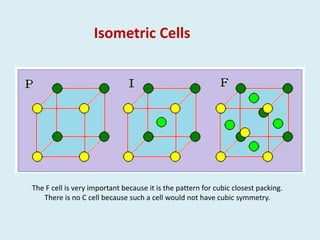
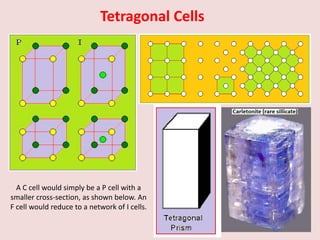
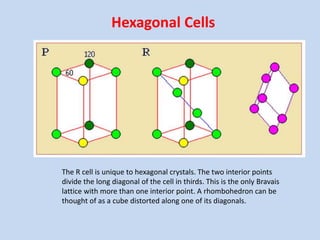
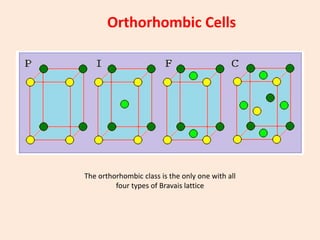
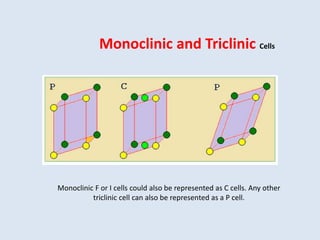
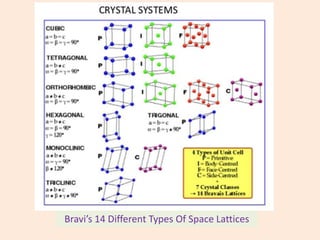
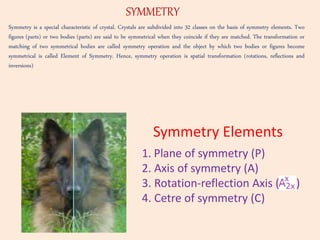
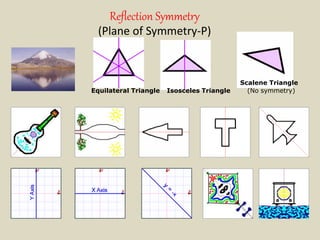
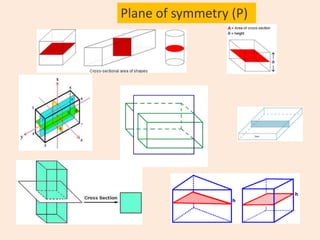
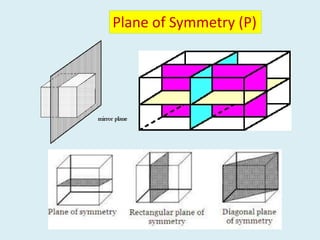
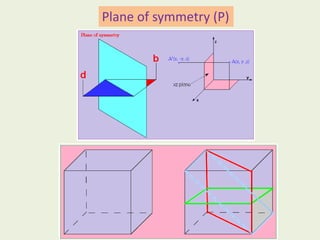
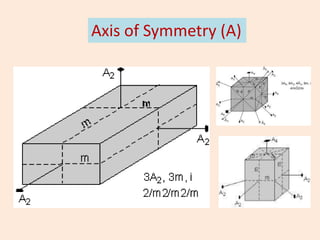
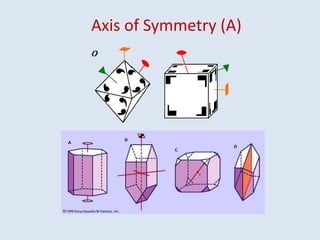
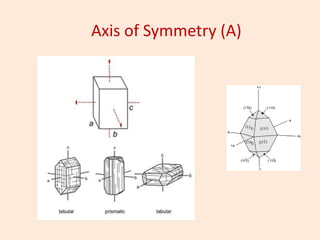
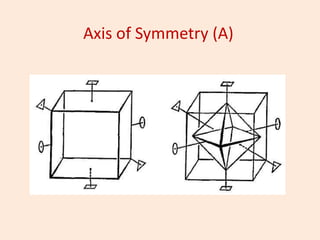
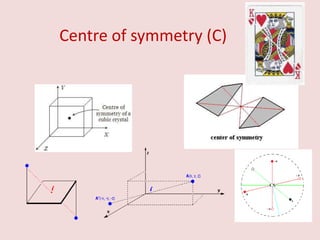
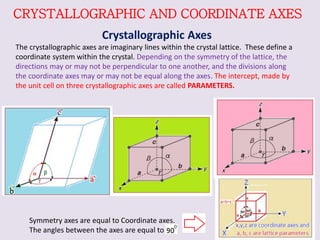
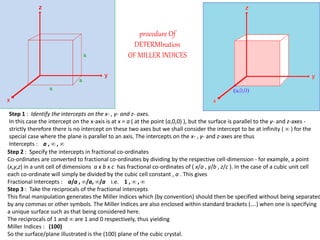
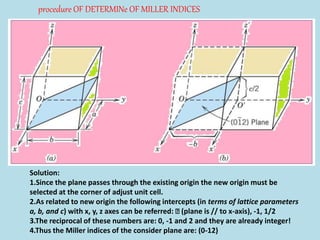
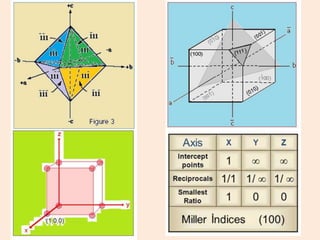
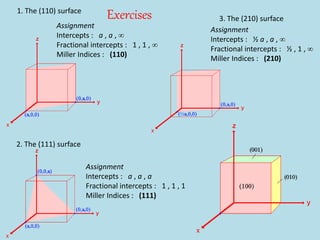
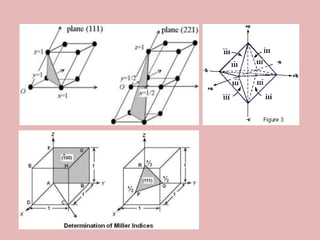
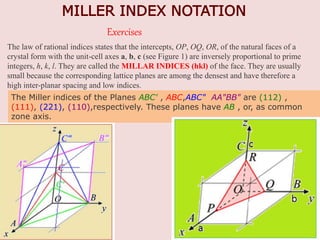
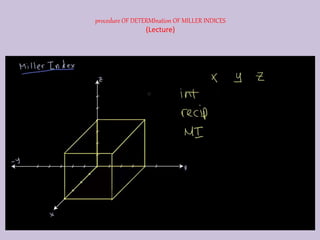
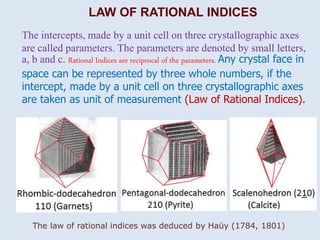
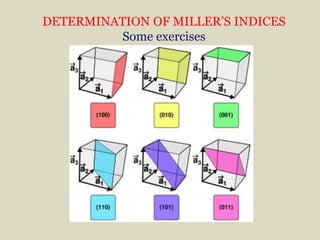
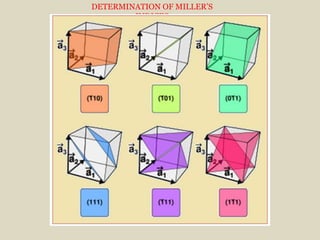
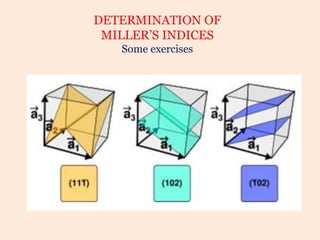
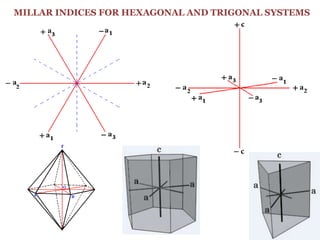
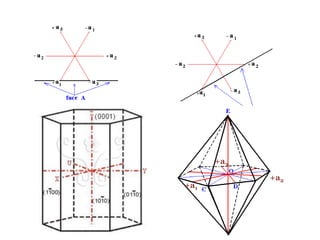
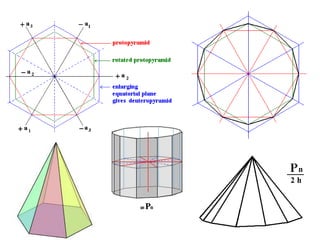
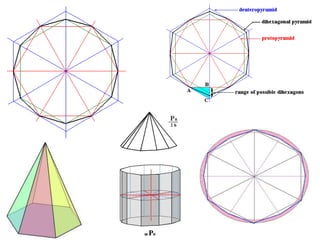
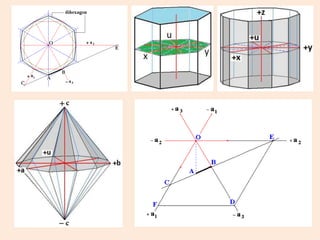
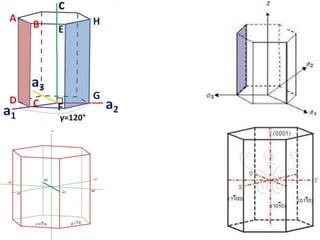
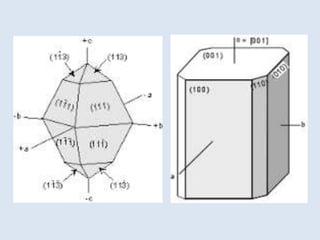
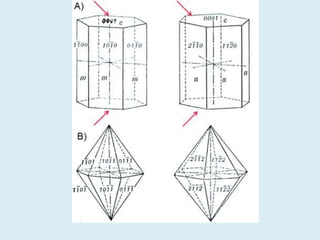
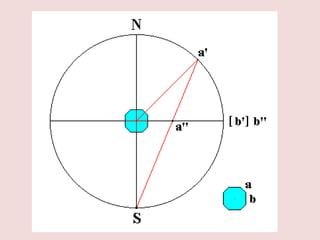
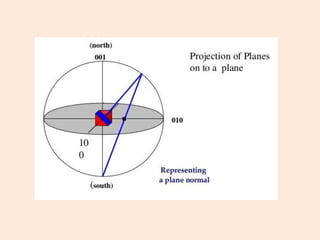
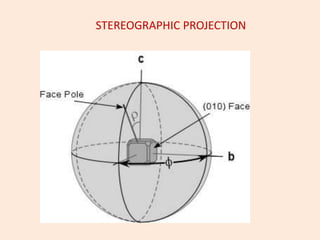
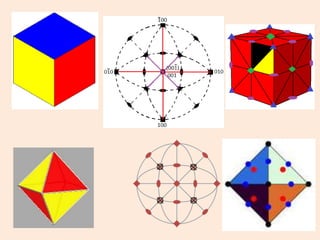
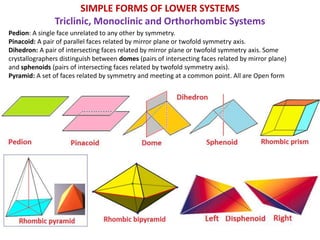
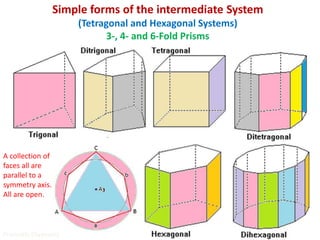
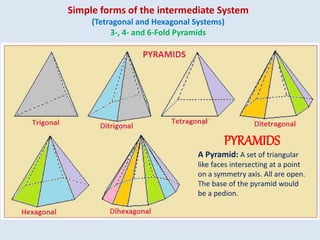
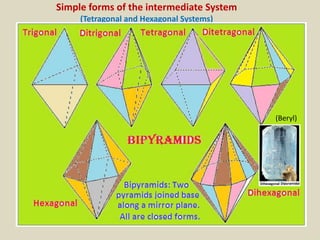
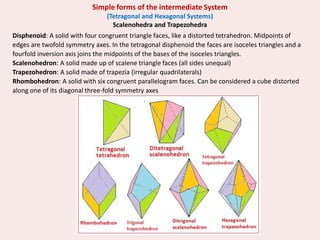
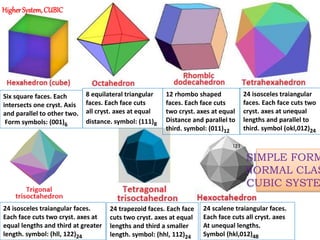
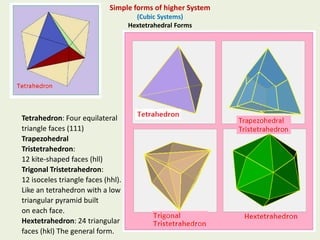
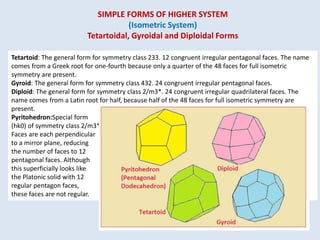
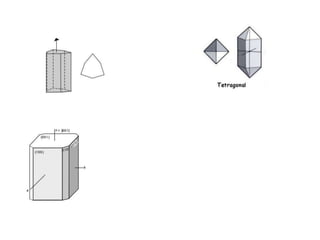
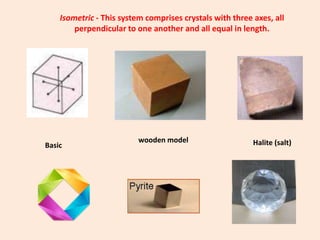
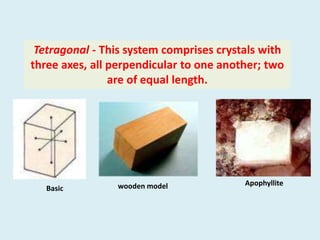
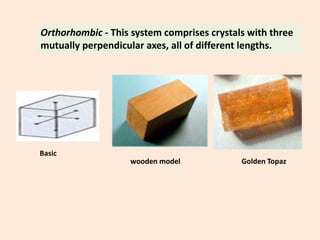
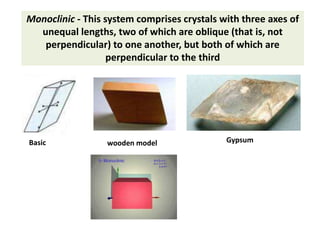
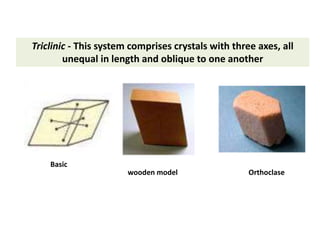
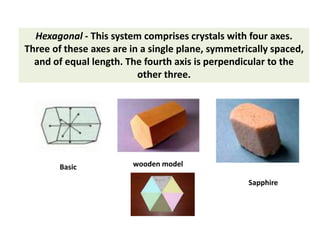
Crystallography is the branch of science that studies crystals, their growth and structure. It deals with crystals' external form, internal arrangement of atoms, and physical properties. Crystals have a regularly repeating internal structure. Miller indices are a notation system used to describe crystal planes and directions within a crystal lattice by three integers h, k, l. The law of rational indices states that the intercepts made by a crystal plane with the unit cell axes are inversely proportional to integers that become the Miller indices. Determining Miller indices involves finding intercepts with axes, converting to fractional coordinates, and taking reciprocals.