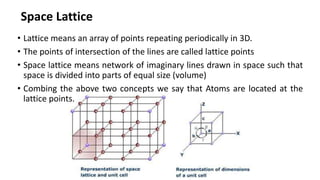
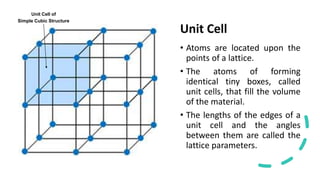
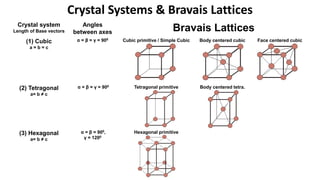
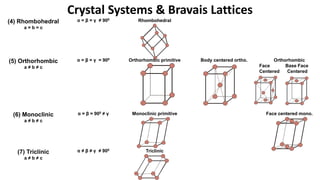
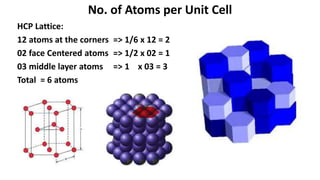
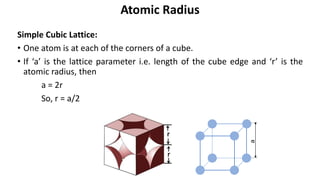
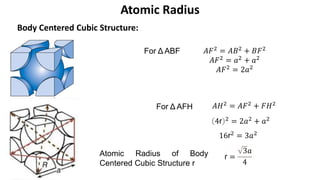
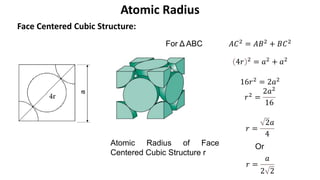
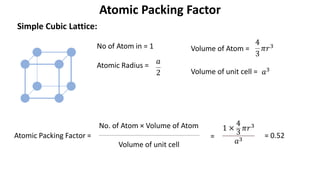
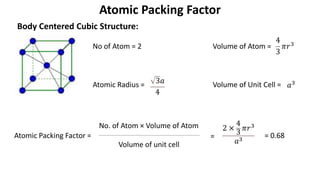
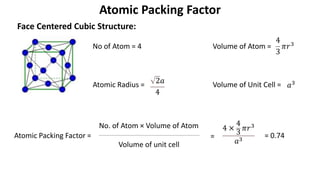
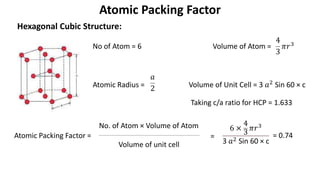
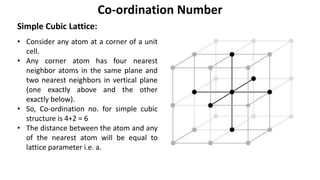
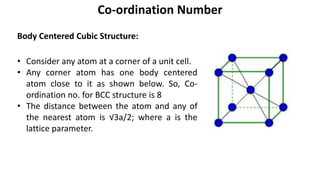
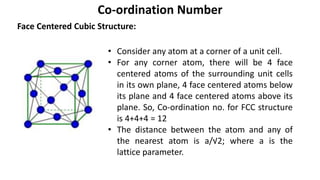
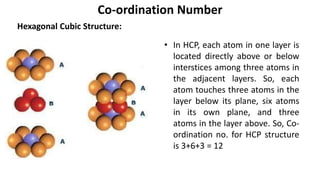
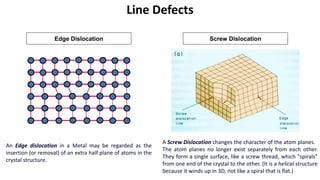
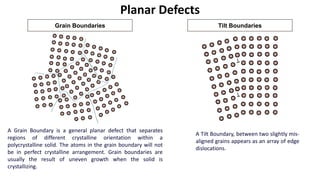
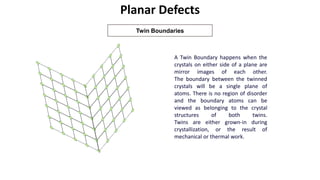
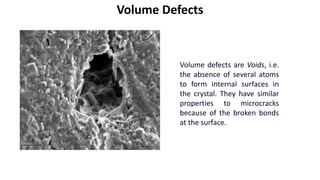
The document summarizes key concepts related to crystal structure:
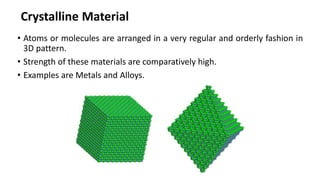
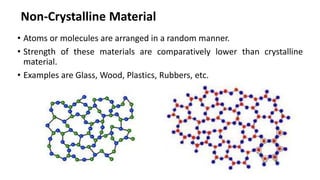
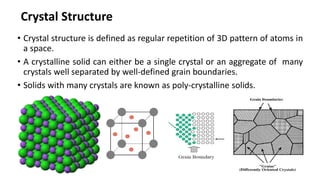
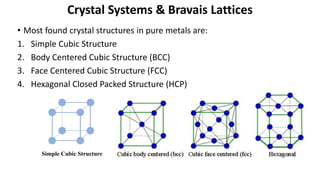
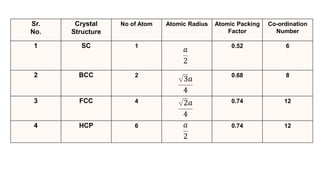
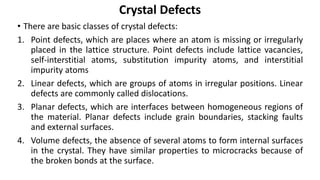
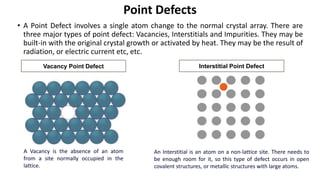
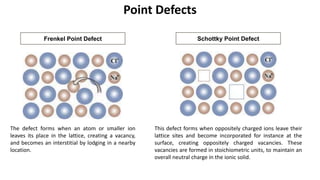
Crystalline materials have atoms or molecules arranged in a regular, orderly 3D pattern which gives them high strength, while non-crystalline materials have a random arrangement and lower strength. A crystal structure is a regular repetition of this 3D pattern defined by a unit cell and space lattice. Common crystal structures include simple cubic, body-centered cubic, face-centered cubic, and hexagonal close-packed. Crystal defects such as point defects, dislocations, grain boundaries, and voids are also discussed.