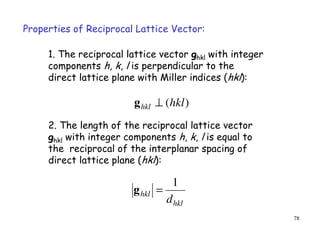
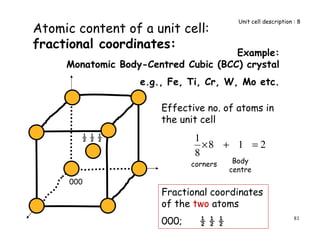
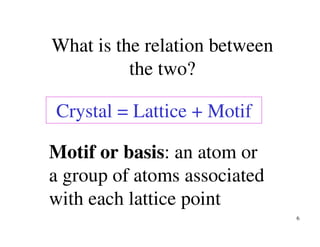
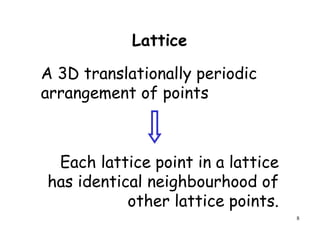
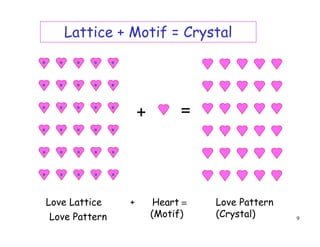
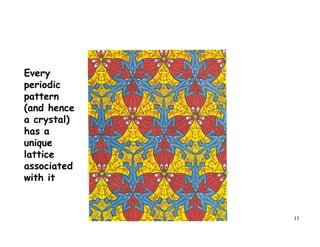
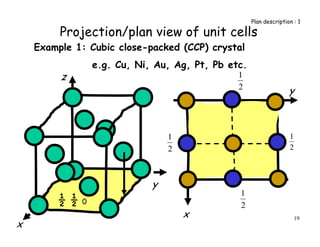
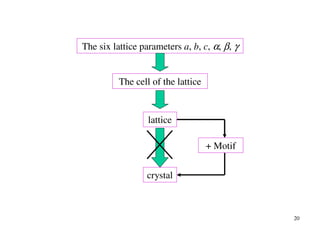
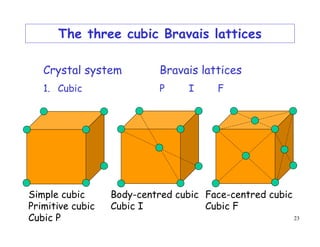
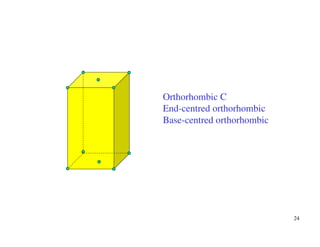
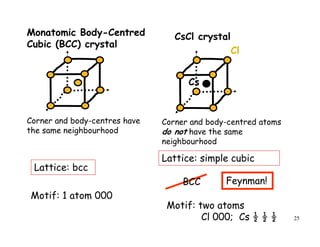
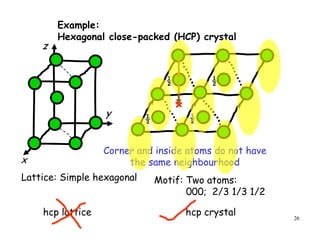
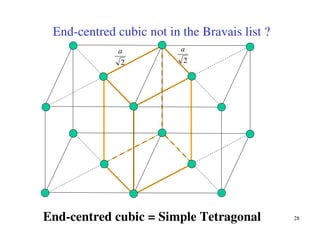
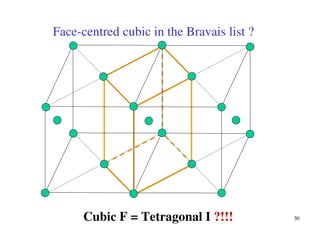
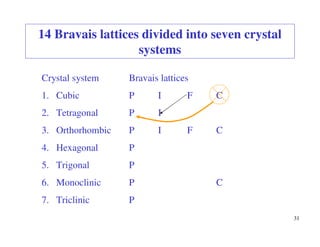
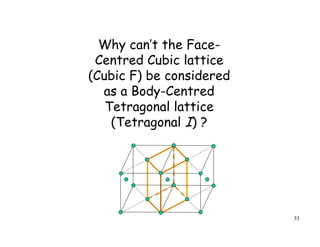
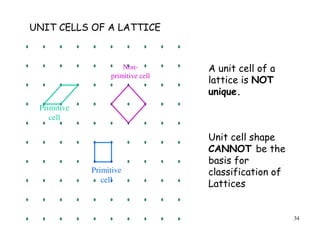
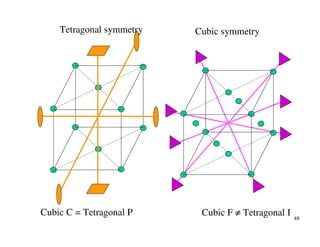
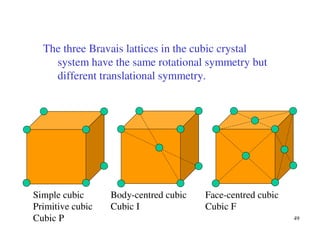
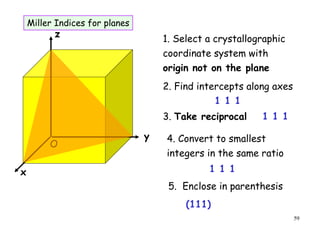
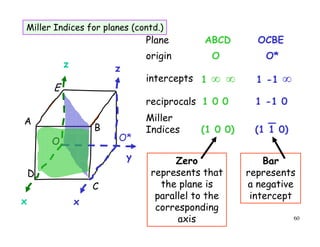
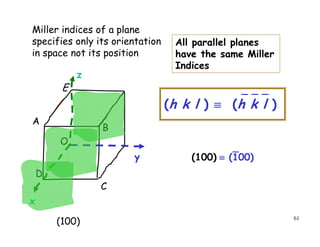
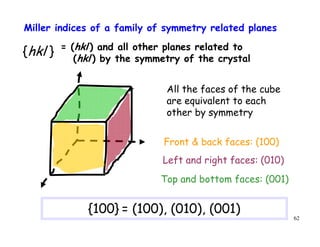
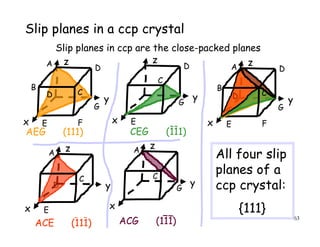
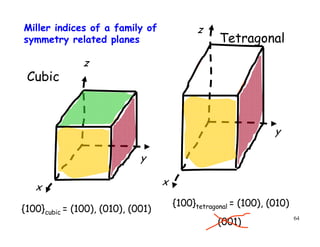
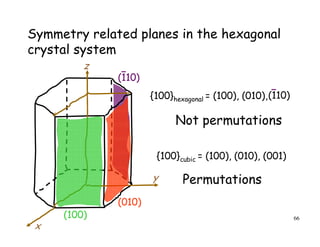
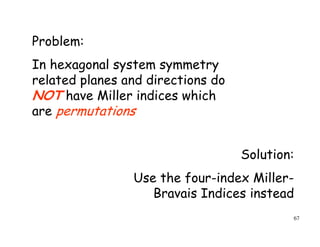
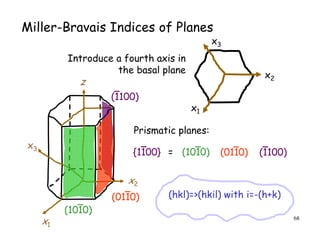
The document provides an introduction to basic crystallography concepts including crystals, lattices, motifs, unit cells, and Miller indices. It discusses that a crystal is defined as a periodically arranged structure of atoms or molecules in space. A lattice refers to the underlying periodic arrangement of points, while a motif represents the atom or group of atoms associated with each lattice point. Crystals are classified into 7 crystal systems and 14 Bravais lattices based on their rotational and translational symmetry properties. Miller indices are used to describe directions and planes in a crystal lattice using small integers.
























![53
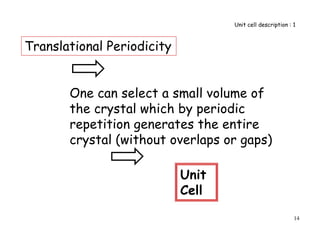
1. Choose a point on the direction
as the origin.
2. Choose a coordinate system
with axes parallel to the unit
cell edges.
x
y 3. Find the coordinates of
another point on the direction in
terms of a, b and c
4. Reduce the coordinates to smallest integers.
5. Put in square brackets
Miller Indices of Directions
[100]
1a+0b+0c
z
1, 0, 0
1, 0, 0
Miller Indices 2
a
b
c](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-53-320.jpg)
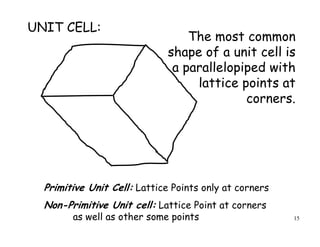
![54
y
z
Miller indices of a direction:
only the orientation
not its position or sense
All parallel directions have the
same Miller indices
[100]x
Miller Indices 3](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-54-320.jpg)
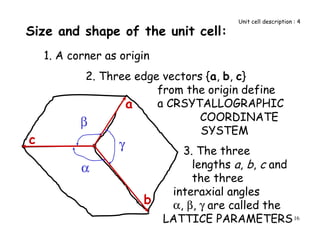
![55
x
y
z
O
A
1/2, 1/2, 1
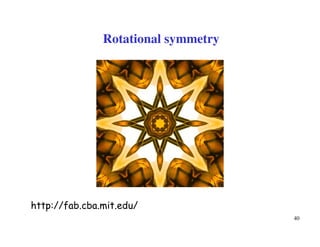
[1 1 2]
OA=1/2 a + 1/2 b + 1 c
P
Q
x
y
z
PQ = -1 a -1 b + 1 c
-1, -1, 1
Miller Indices of
Directions (contd.)
[ 1 1 1 ]
__
-ve steps are shown as bar over the number
Direction OA
Direction PQ](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-55-320.jpg)
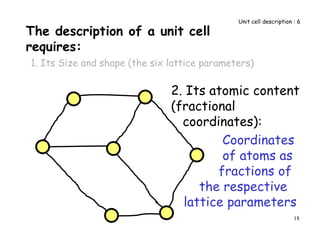
![56
Miller indices of a family of symmetry related directions
[100]
[001]
[010]
uvw = [uvw] and all other directions related to
[uvw] by the symmetry of the crystal
cubic
100 = [100], [010],
[001]
tetragonal
100 = [100], [010]
Cubic
Tetragonal
[010]
[100]
Miller Indices 4](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-56-320.jpg)

![58
Miller indices of slip directions in CCP
y
z
[110]
[110]
[101]
[ 101]
[011]
[101]
Slip directions = close-packed directions = face diagonals
x
Six slip directions:
[110]
[101]
[011]
[110]
[101]
[101]
All six slip
directions in ccp:
110
Miller Indices 5](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-58-320.jpg)
![65
[100]
[010]
Symmetry related directions in the
hexagonal crystal system
cubic
100 = [100], [010], [001]
hexagonal
100
Not permutations
Permutations
[110]
x
y
z
= [100], [010], [110]](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-65-320.jpg)
![69
Miller-Bravais Indices of Directions in
hexagonal crystals
x1
x2
x3
Basal plane
=slip plane
=(0001)
[uvw]=>[UVTW]
wWvuTuvVvuU =+−=−=−= );();2(
3
1
);2(
3
1
Require that: 0=++ TVU
Vectorially 0aaa 21 =++ 3
caa
caaa
wvu
WTVU
++=
+++
21
321
a1
a2
a2](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-69-320.jpg)
![70
a1
a1
-a2
-a3
]0112[
a2
a3
x1
x2
x3
wWvuTuvVvuU =+−=−=−= );();2(
3
1
);2(
3
1
[ ] ]0112[0]100[ 3
1
3
1
3
2
≡−−⇒
[ ] ]0121[0]010[ 3
1
3
2
3
1
≡−−⇒
[ ] ]2011[0]011[ 3
2
3
1
3
1
≡−−⇒
x1:
x2:
x3:
Slip directions in hcp
0112
Miller-Bravais indices of slip directions in hcp
crystal:](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-70-320.jpg)
![71
Some IMPORTANT Results
Condition for a direction [uvw] to be parallel to a plane or lie in
the plane (hkl):
h u + k v + l w = 0
Weiss zone law
True for ALL crystal systems
h U + k V + i T +l W = 0](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-71-320.jpg)
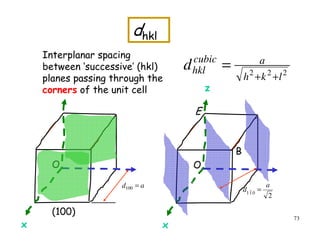
![72
CUBIC CRYSTALS
[hkl] ⊥⊥⊥⊥ (hkl)
Angle between two directions [h1k1l1] and [h2k2l2]:
C
[111]
(111)
2
2
2
2
2
2
2
1
2
1
2
1
212121
cos
lkhlkh
llkkhh
++++
++
=θ](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-72-320.jpg)
![74
[uvw] Miller indices of a direction (i.e. a set of
parallel directions)
<uvw> Miller indices of a family of symmetry
related directions
(hkl) Miller Indices of a plane (i.e. a set of
parallel planes)
{hkl} Miller indices of a family of symmetry
related planes
[uvtw] Miller-Bravais indices of a direction,
(hkil) plane in a hexagonal system
Summary of Notation convention for Indices](https://image.slidesharecdn.com/basiccrystallography-150413032307-conversion-gate01/85/Basic-crystallography-74-320.jpg)