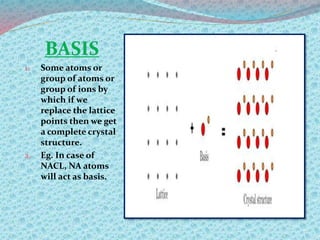
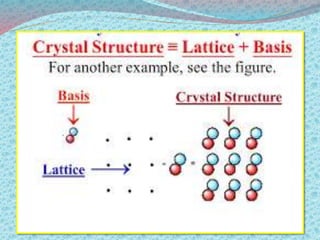
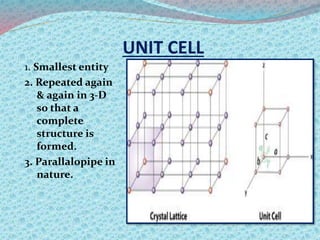
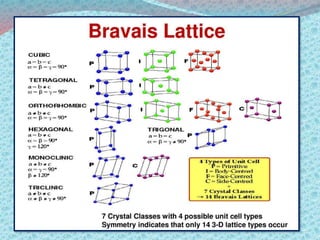
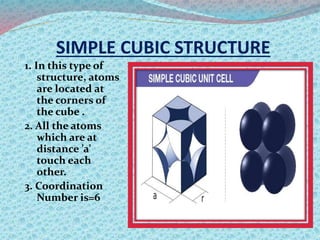
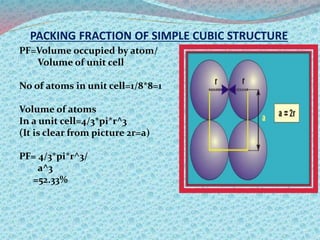
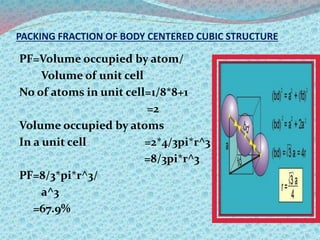
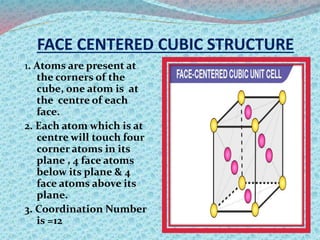
There are two types of materials - crystalline and non-crystalline. Crystalline materials have atoms arranged in a regular pattern, making them anisotropic, while non-crystalline materials have atoms in a random arrangement, making them isotropic. Crystalline materials can exist as polycrystalline, with many regularly ordered grains, or single crystalline, with a single regularly ordered grain. An important concept in crystalline materials is the unit cell, which is the smallest repeating unit that extends in three dimensions to form the entire crystal structure. There are three main types of unit cells - simple cubic, body centered cubic, and face centered cubic - which differ in their atomic arrangements and packing fractions.