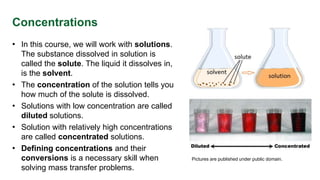
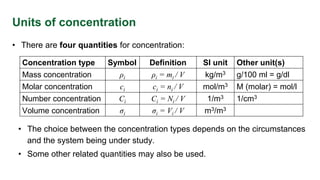
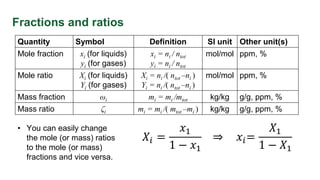
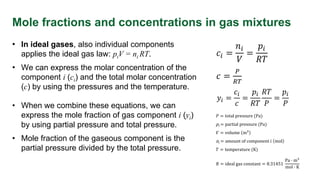
This document provides an overview of solution concentrations, including definitions, types, and relevant calculations essential for mass transfer problems. It details four types of concentration: mass concentration, molar concentration, number concentration, and volume concentration, along with related quantities such as mole fraction and mass fraction. The content is funded by the European Union's Horizon 2020 program and includes references and links to additional resources.