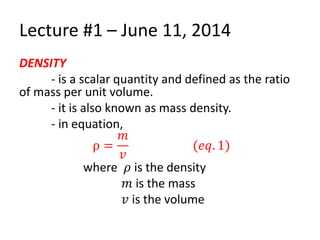
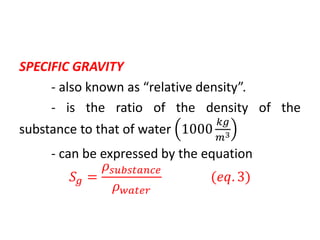
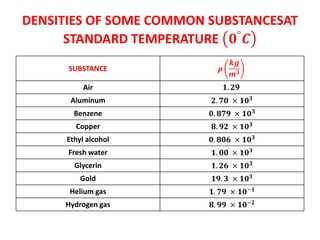
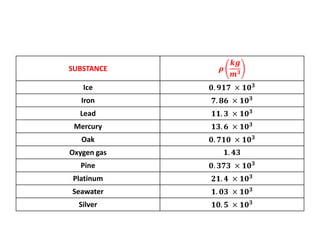
The document discusses density and specific gravity. It defines density as the ratio of mass to volume and gives the equation ρ = m/v. Specific gravity is defined as the ratio of a substance's density to that of water. Example densities are given for common substances like air, water, gold and others. Sample problems demonstrate calculating density, mass, volume and specific gravity using the relevant equations and given values.