Embed presentation
Downloaded 120 times
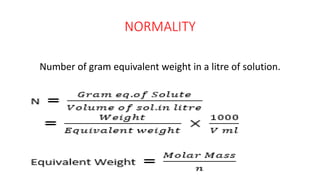

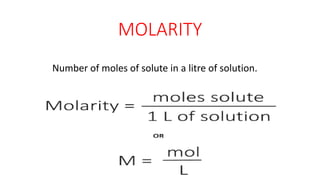

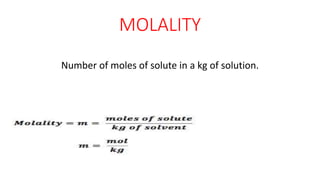





This document defines and compares several units used to express the concentration of solutions: normality is the number of gram equivalent weights in a liter of solution; molarity is the number of moles of solute per liter of solution; molality is the number of moles of solute per kilogram of solution; mole fraction is the ratio of moles of a solute to the total moles of all components; and formality is the number of gram formula mass of an ionic solute in a liter of solution. The document was authored by Mayank to explain these concentration units.










