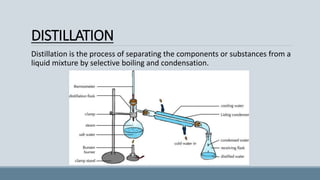
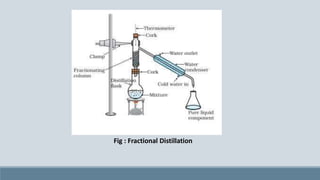
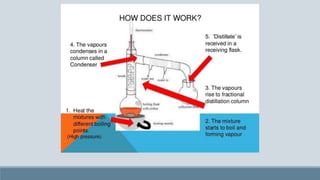
The presentation discusses fractional distillation, a method for separating components from a liquid mixture through selective boiling and condensation, typically using a fractionating column. Key applications include the separation of miscible liquids, alcohol preparation, and organic compound purification. It contrasts fractional distillation with simple distillation, highlighting advantages like efficiency in purifying complex mixtures and disadvantages such as higher energy consumption and complexity.