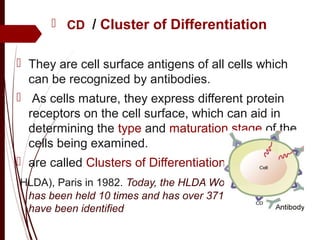
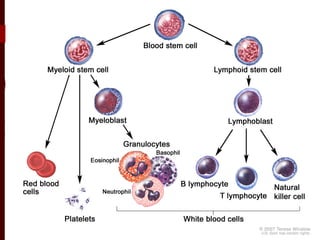
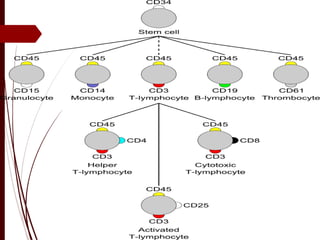
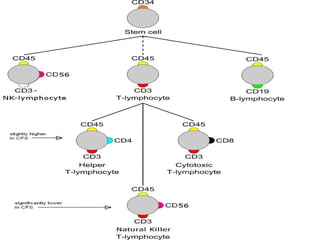
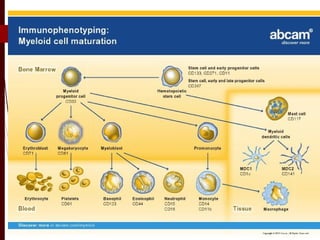
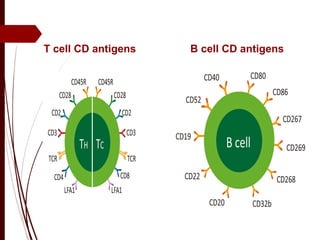
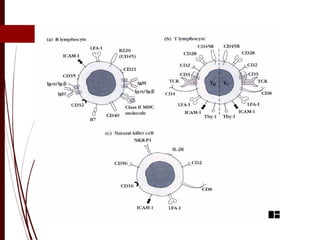
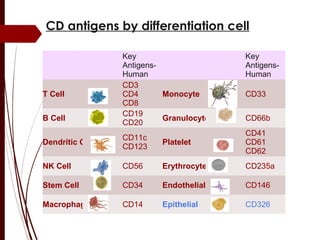
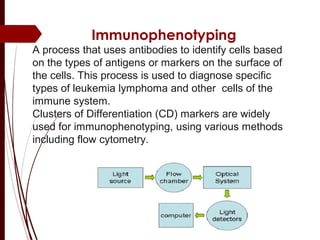
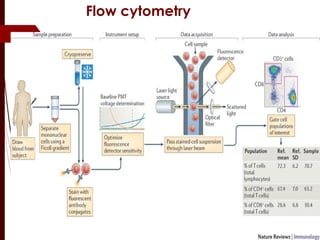
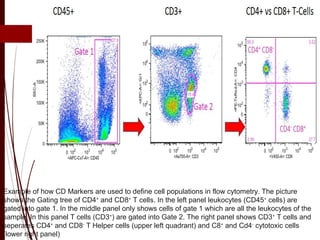
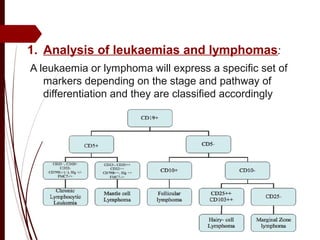
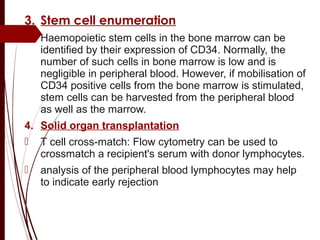
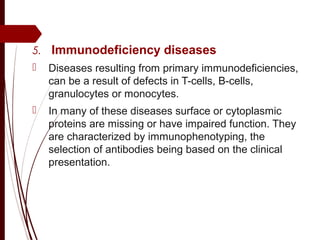
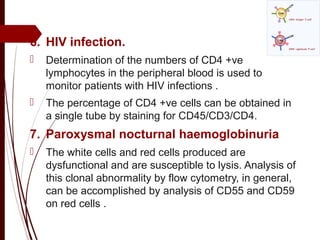
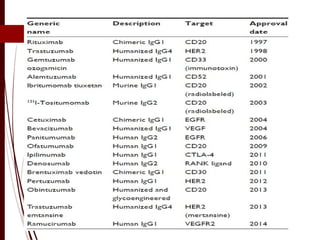
CD antigens, also known as clusters of differentiation, are cell surface proteins that are used to identify and characterize cell types. Over 371 CD markers have been identified that serve important functions like cell signaling. CD markers are analyzed using techniques like flow cytometry and help diagnose diseases like leukemia by identifying abnormal levels of specific CD antigens. Key CD markers have been identified for important immune cells like T cells, B cells, granulocytes, and stem cells. Immunophenotyping using CD markers is an important application in diagnosing diseases and monitoring treatment responses.