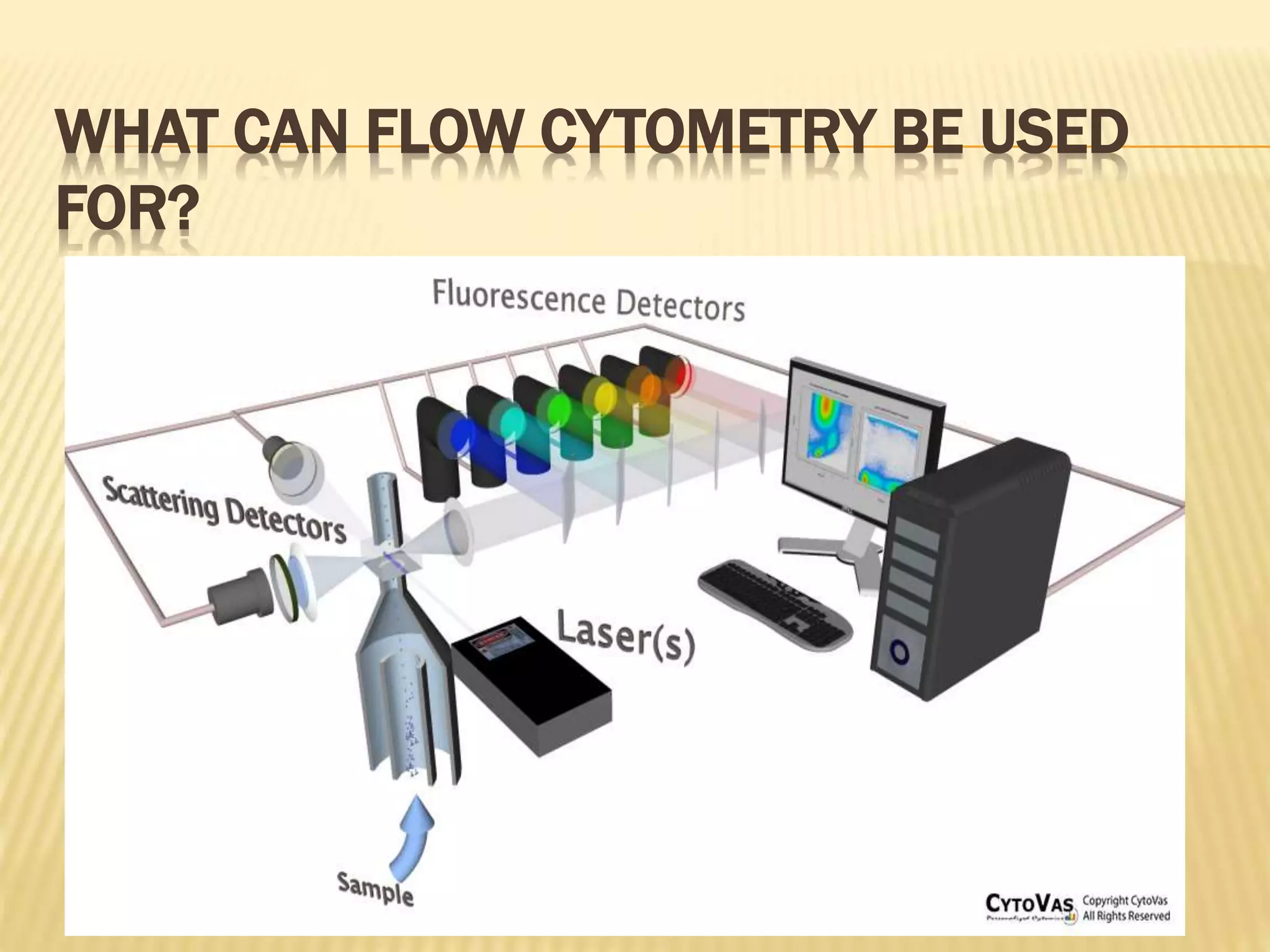
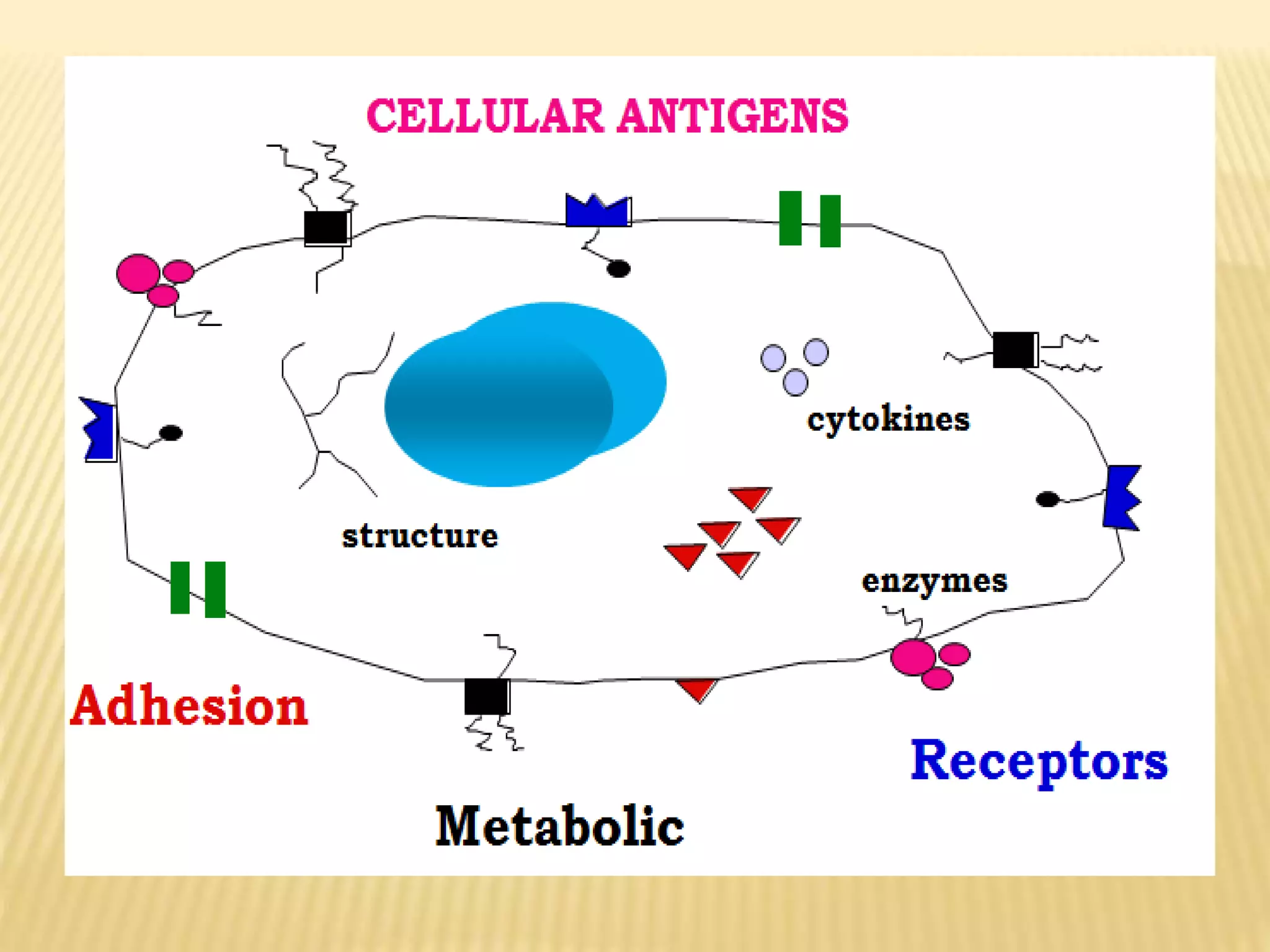
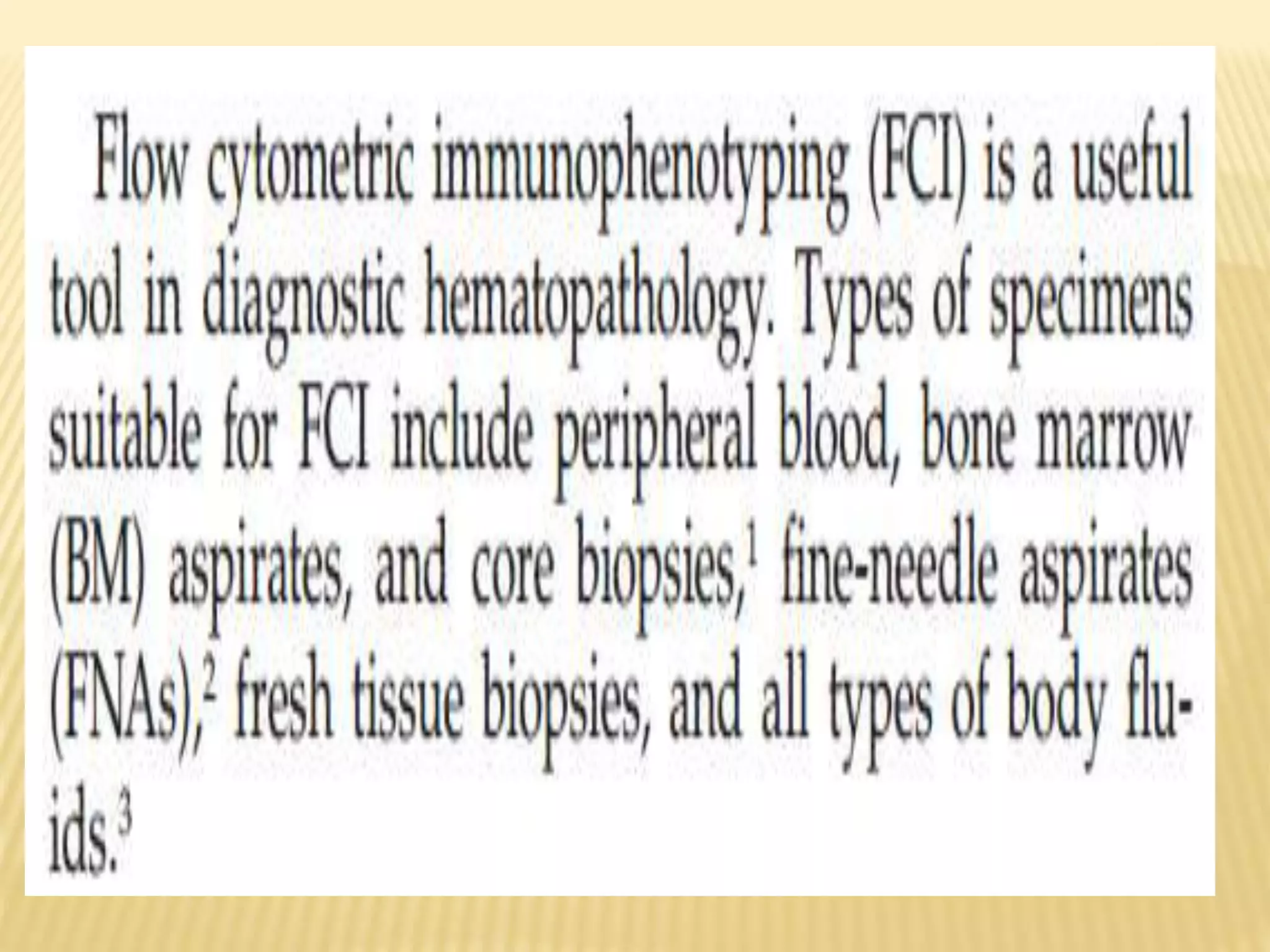
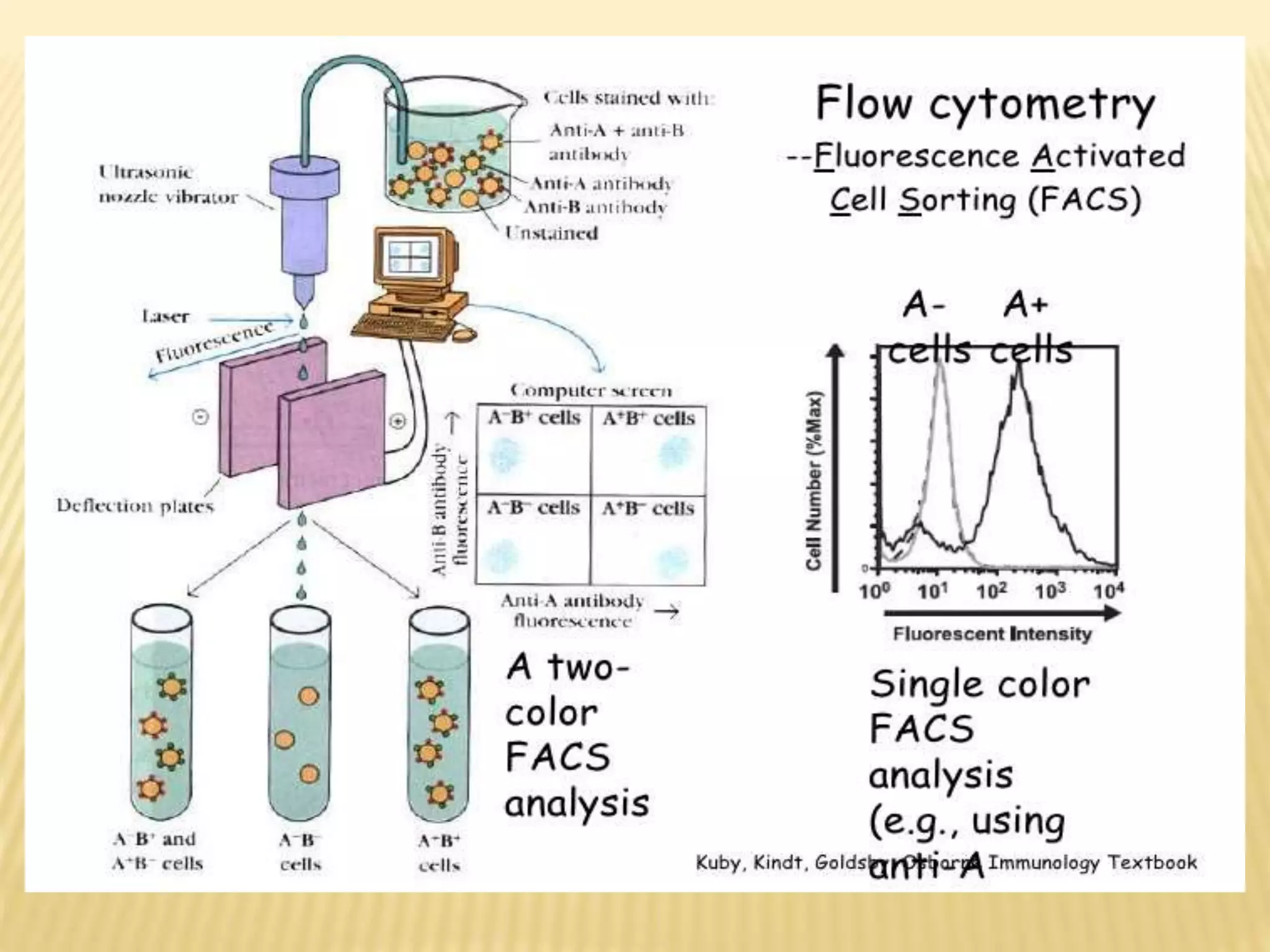
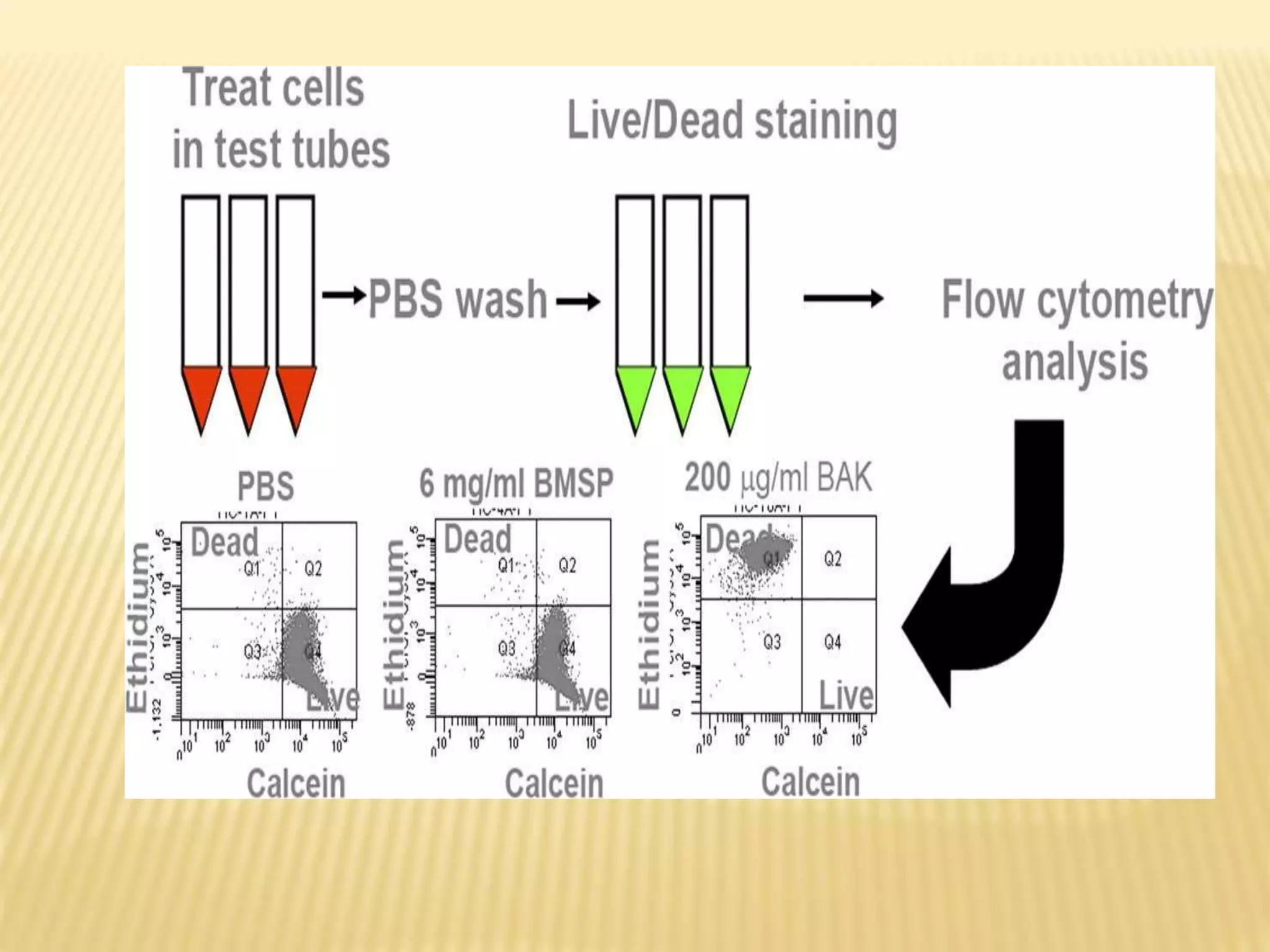
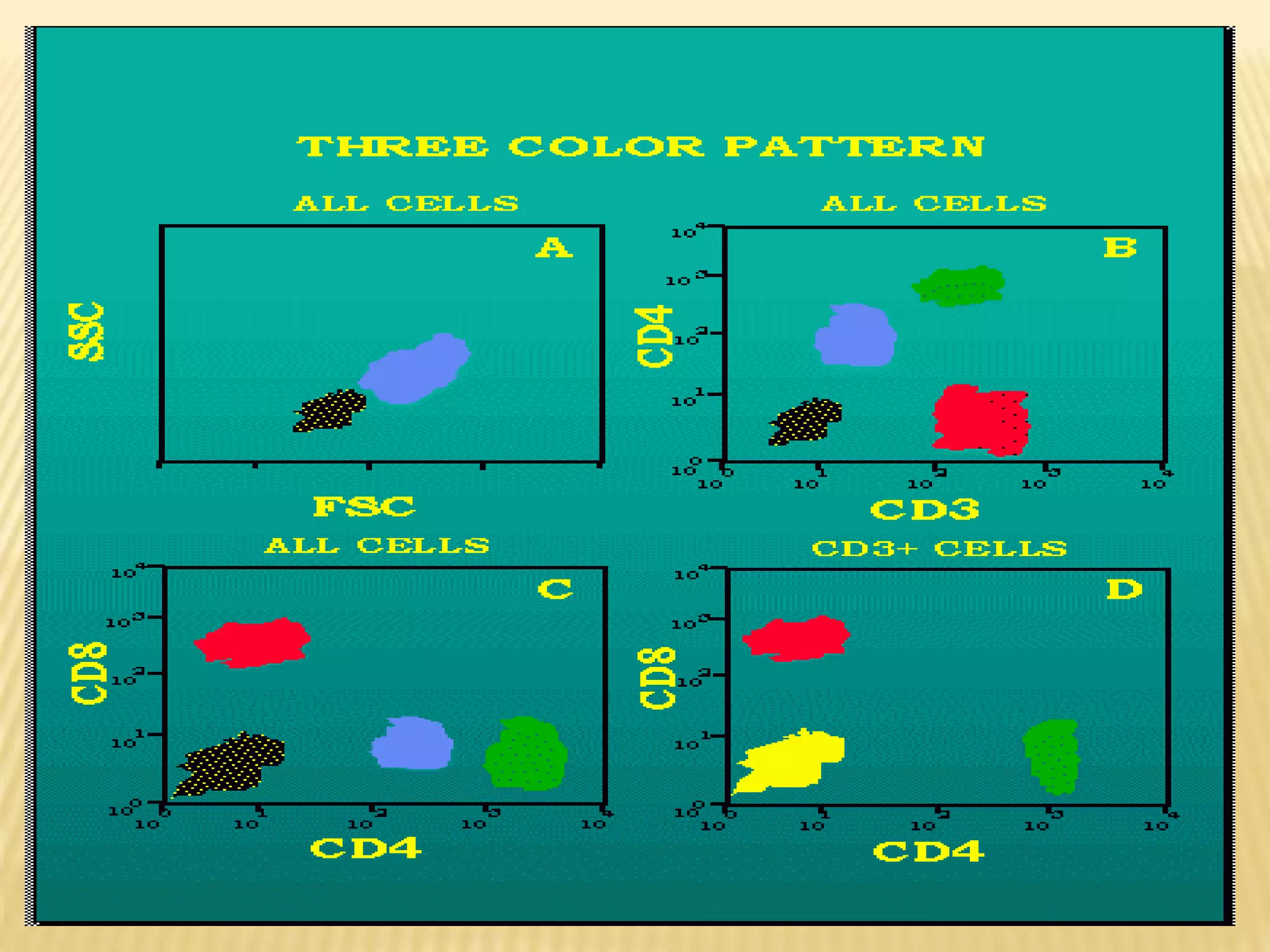
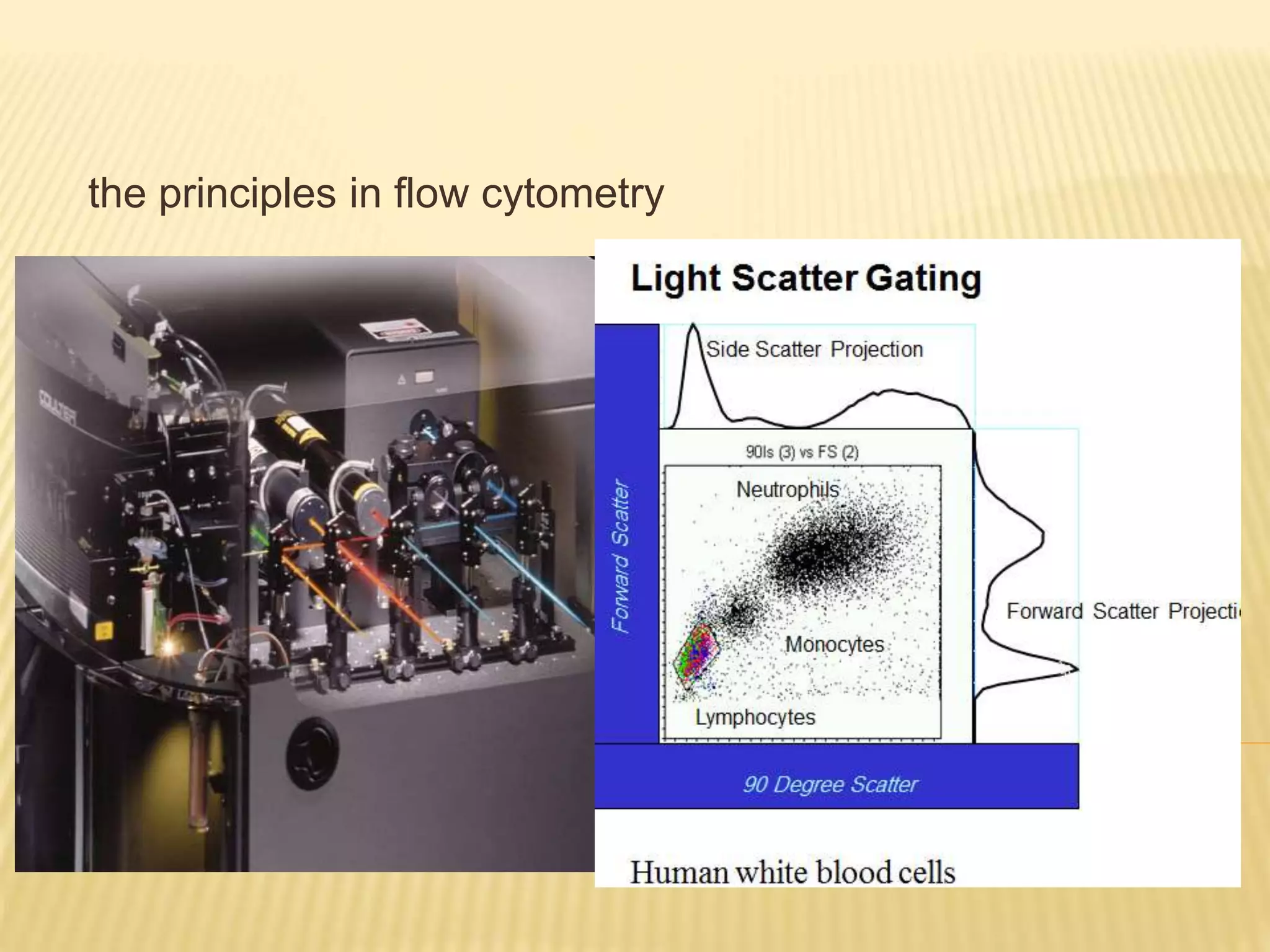
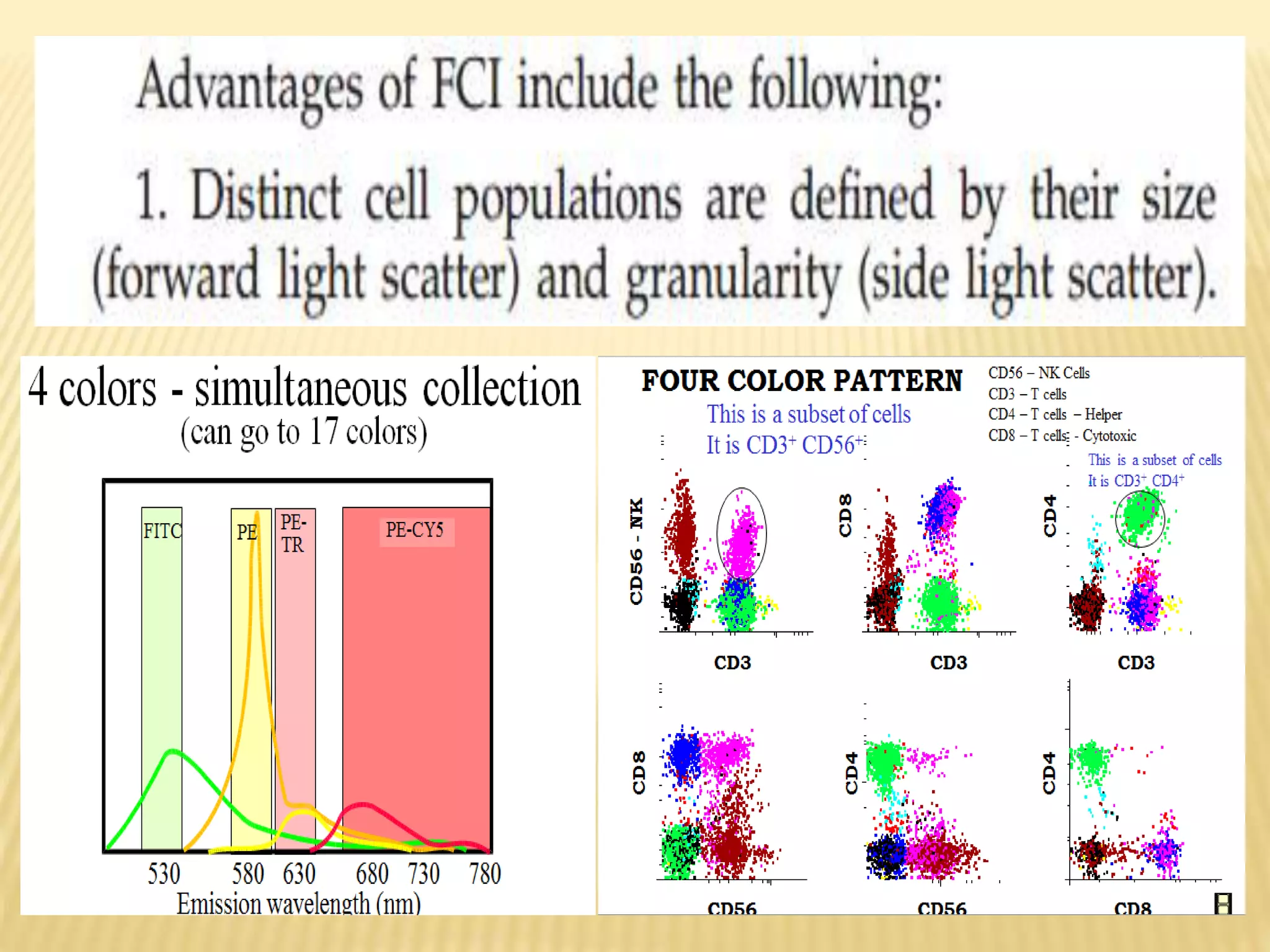
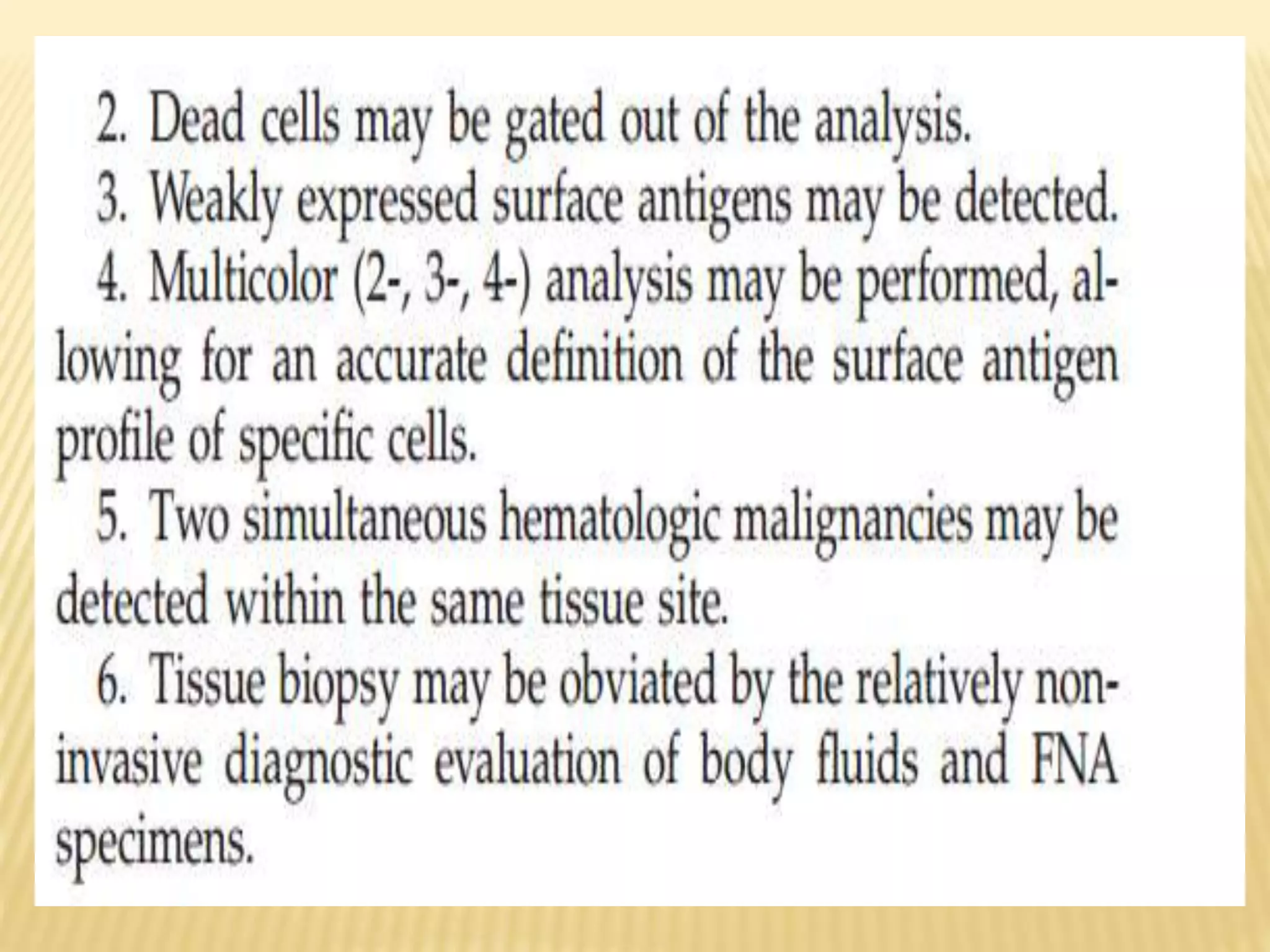
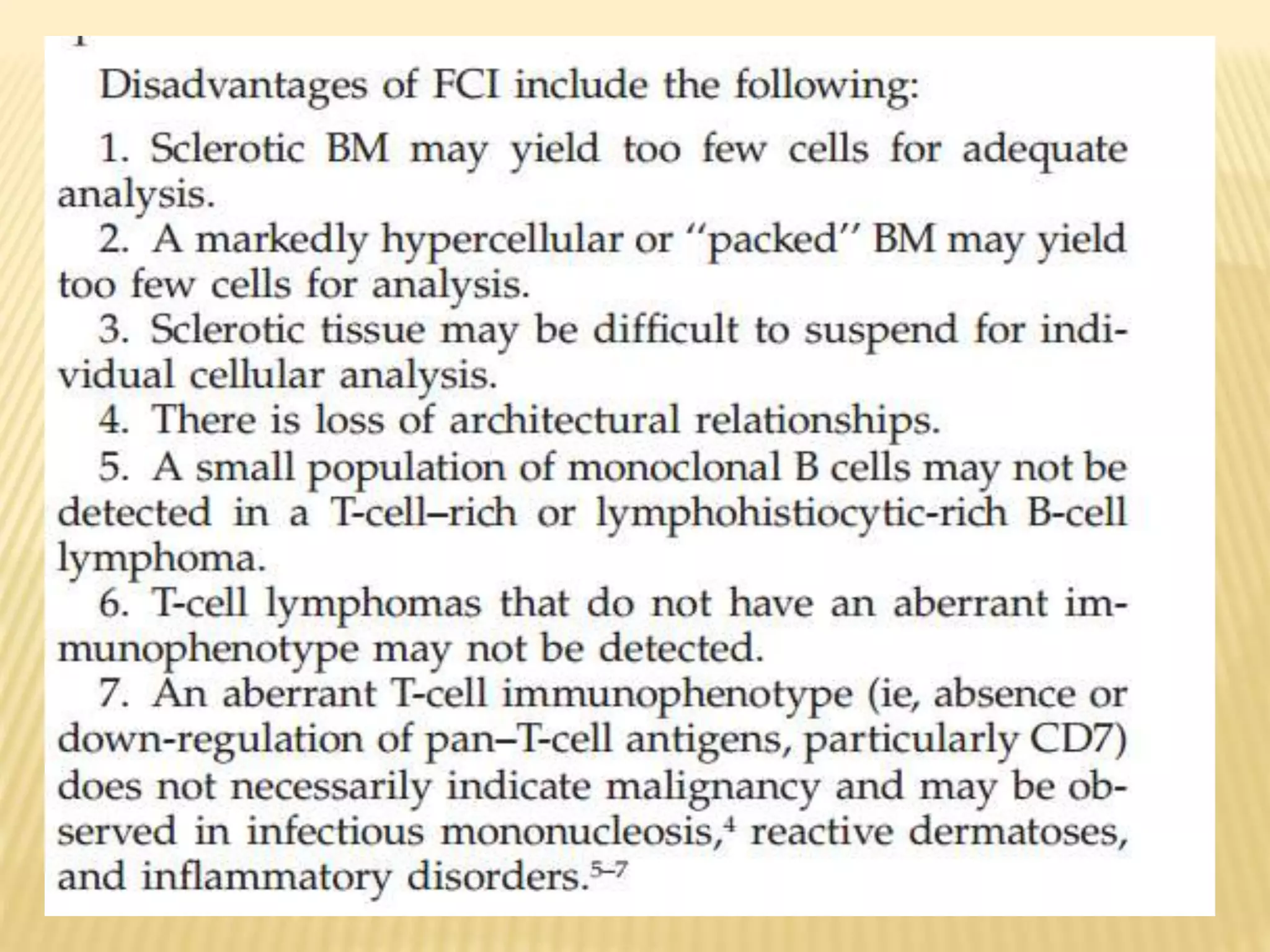
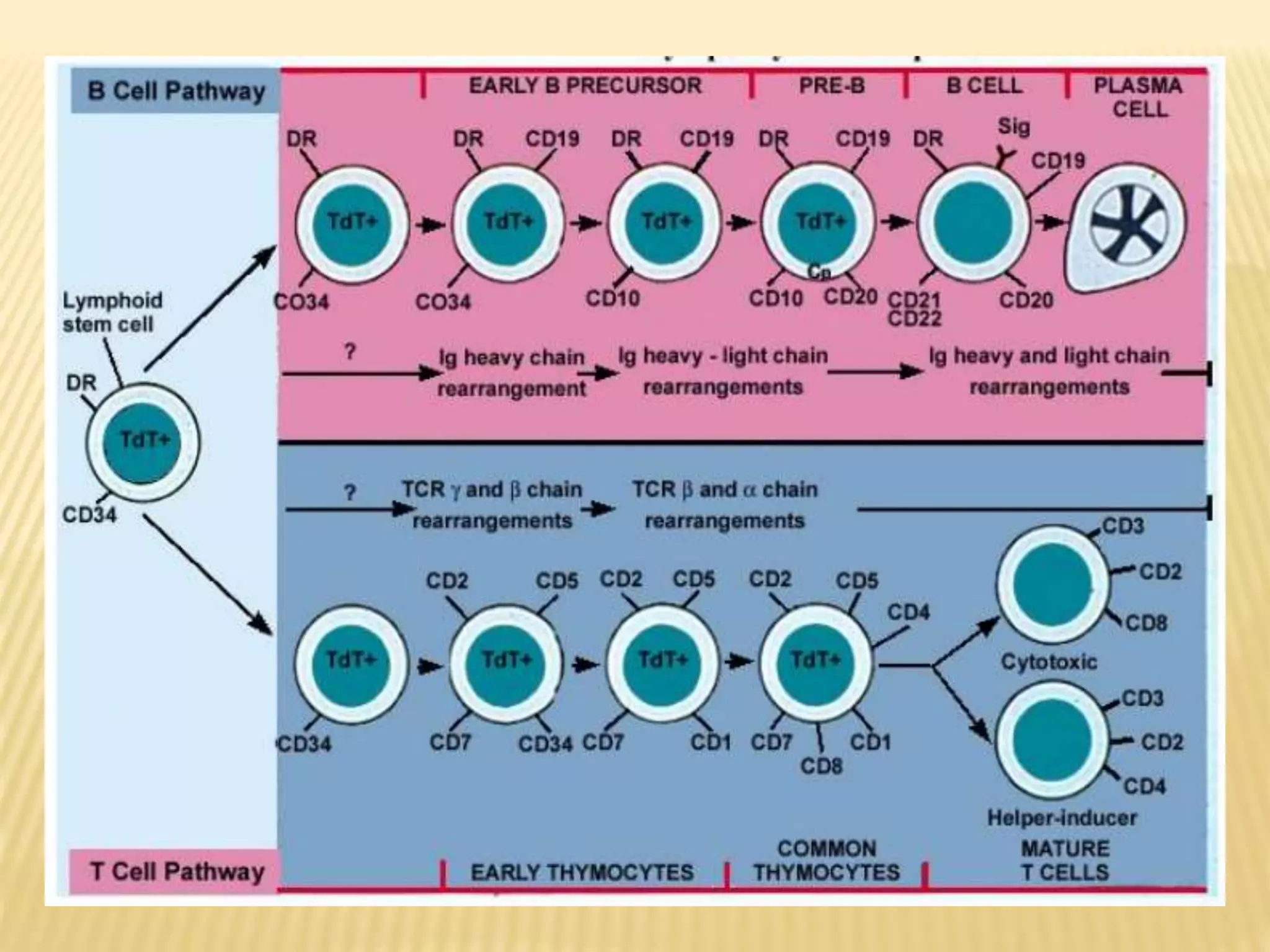
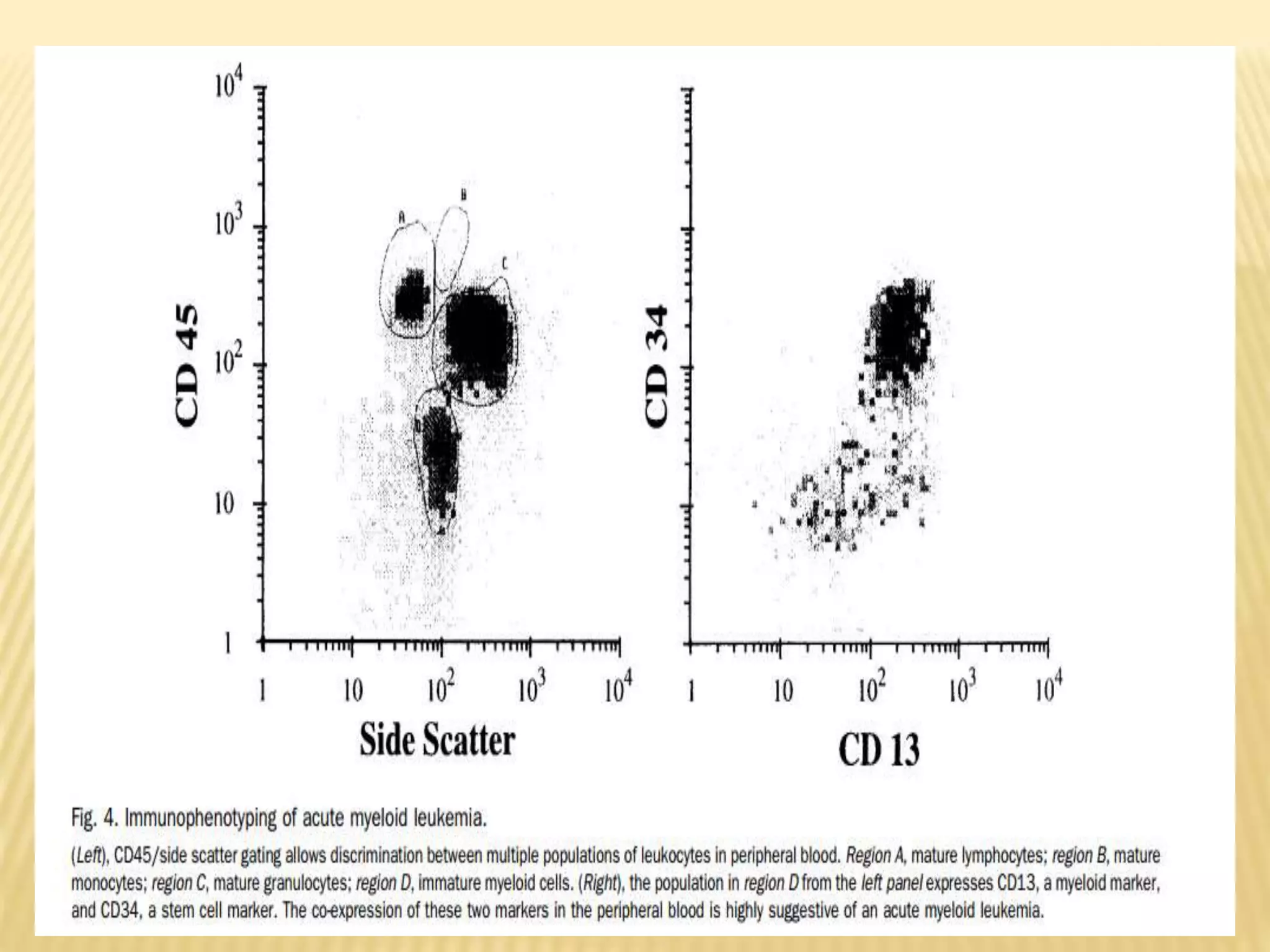
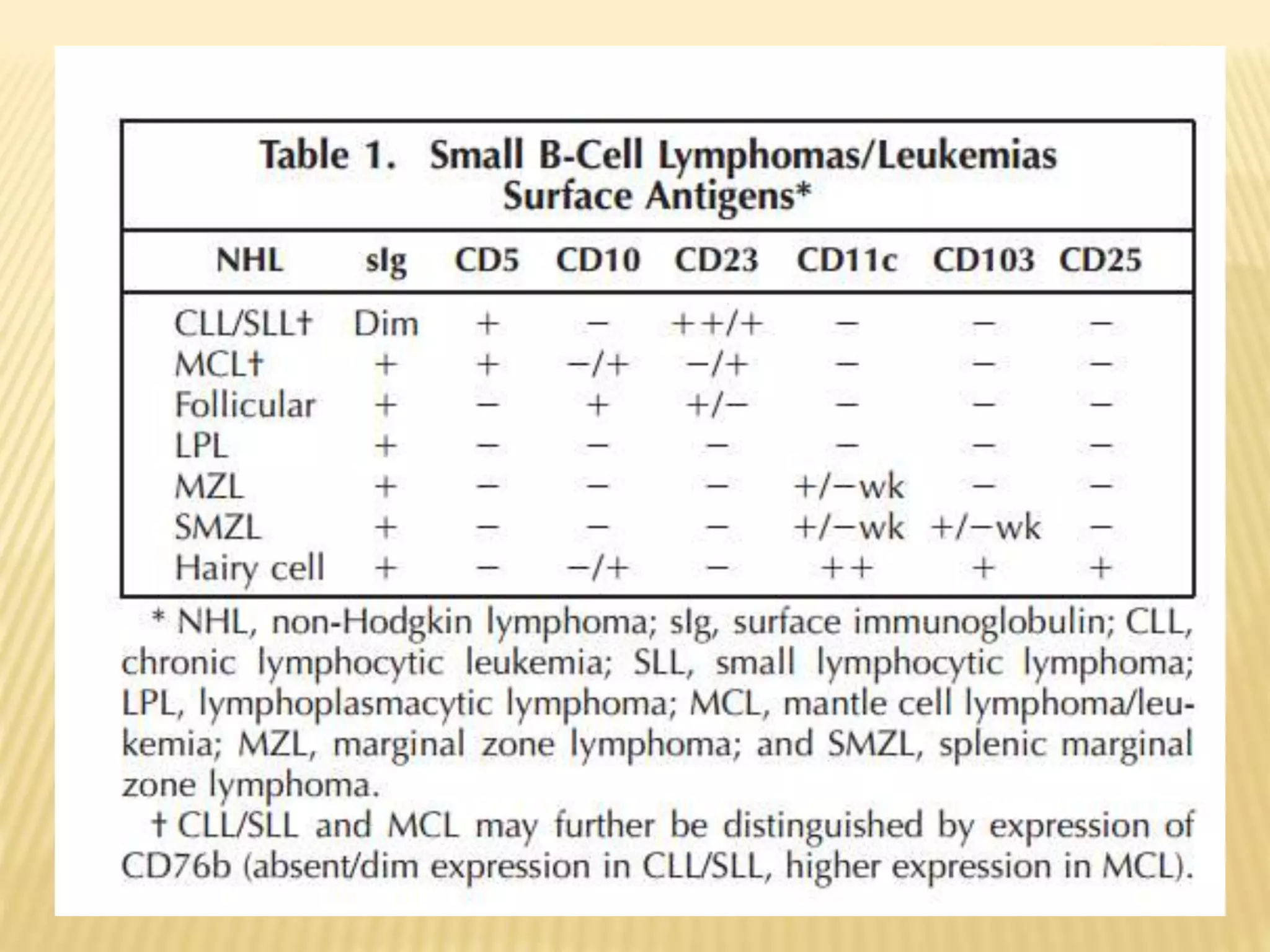
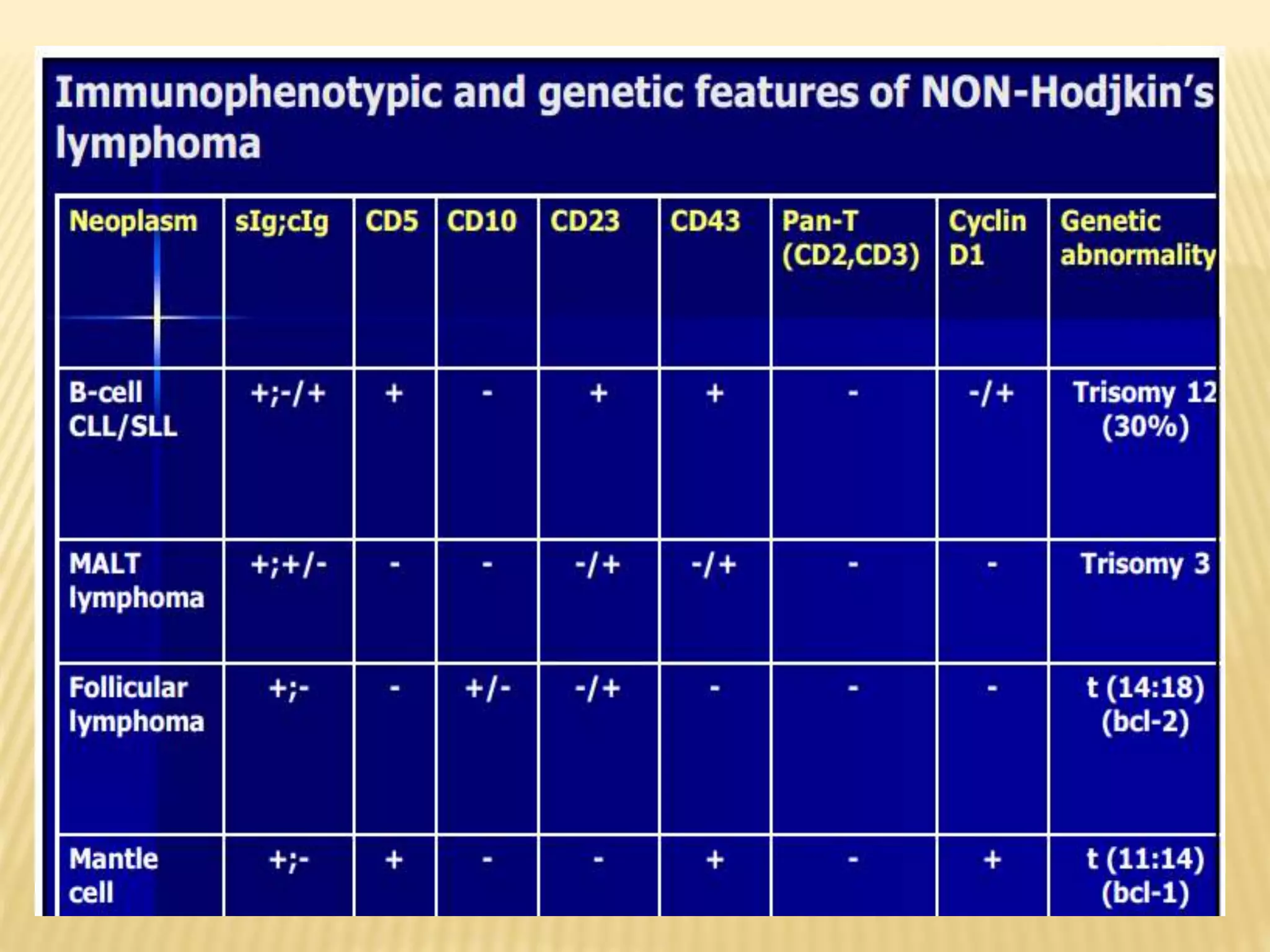
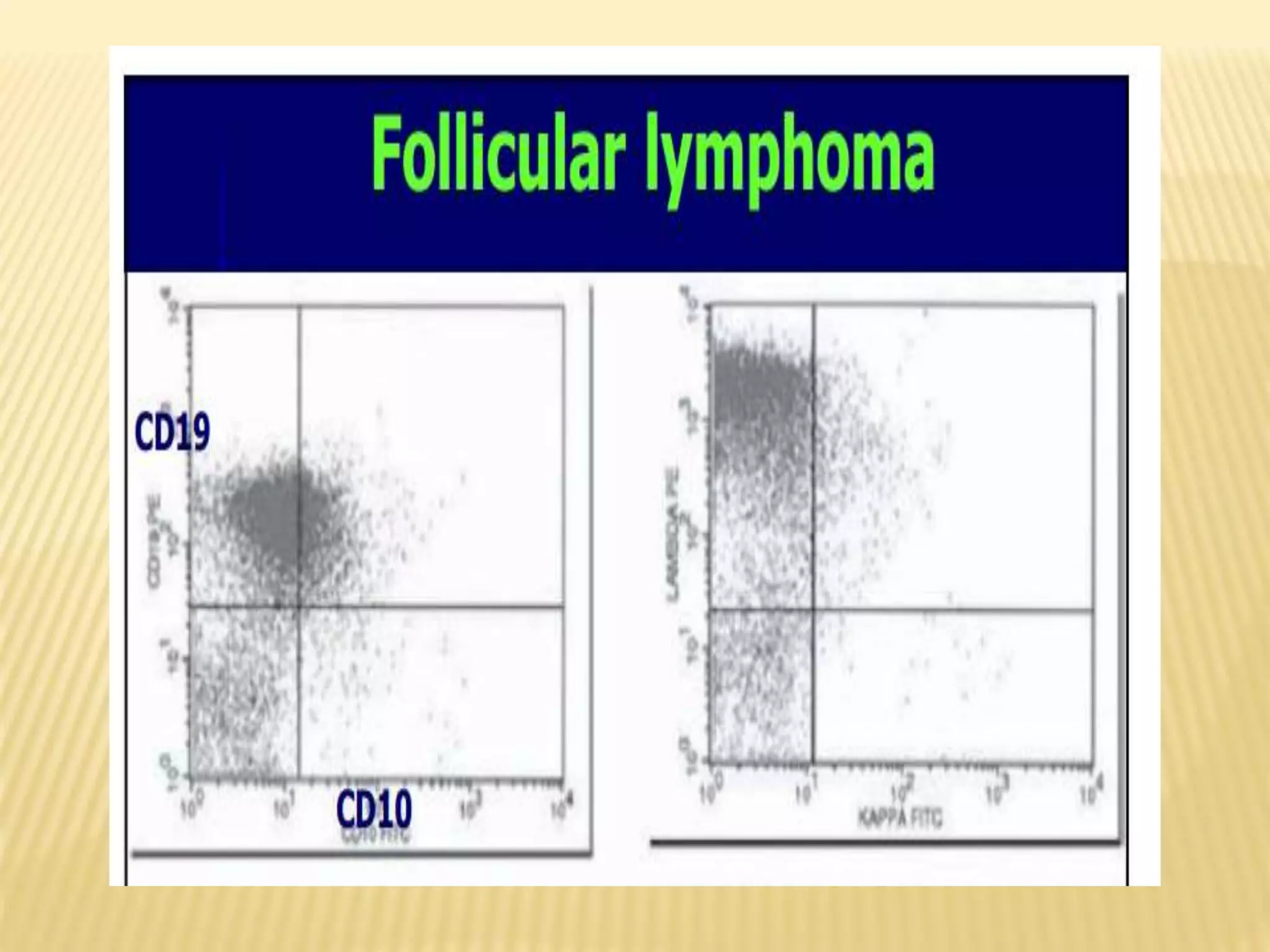
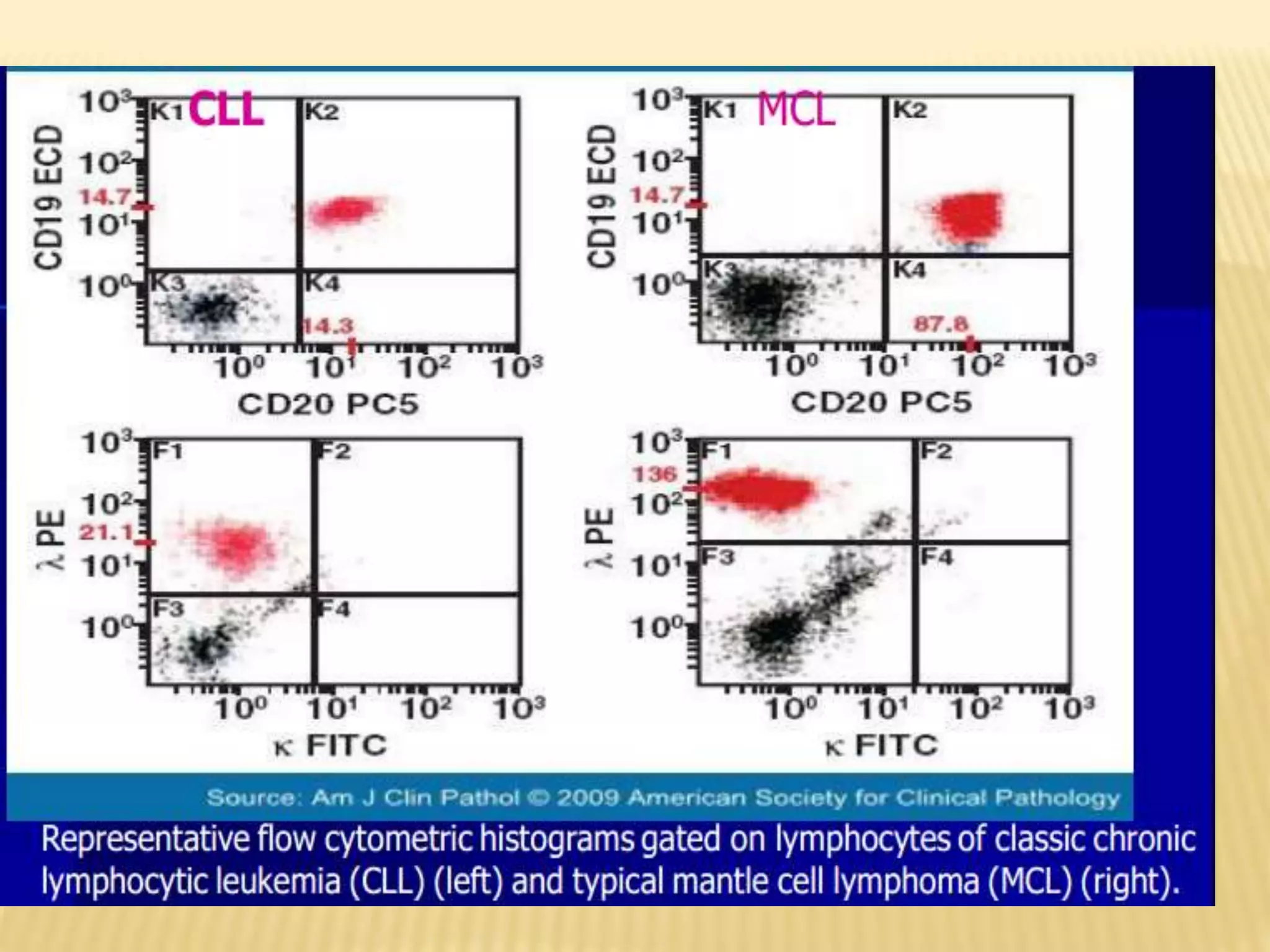
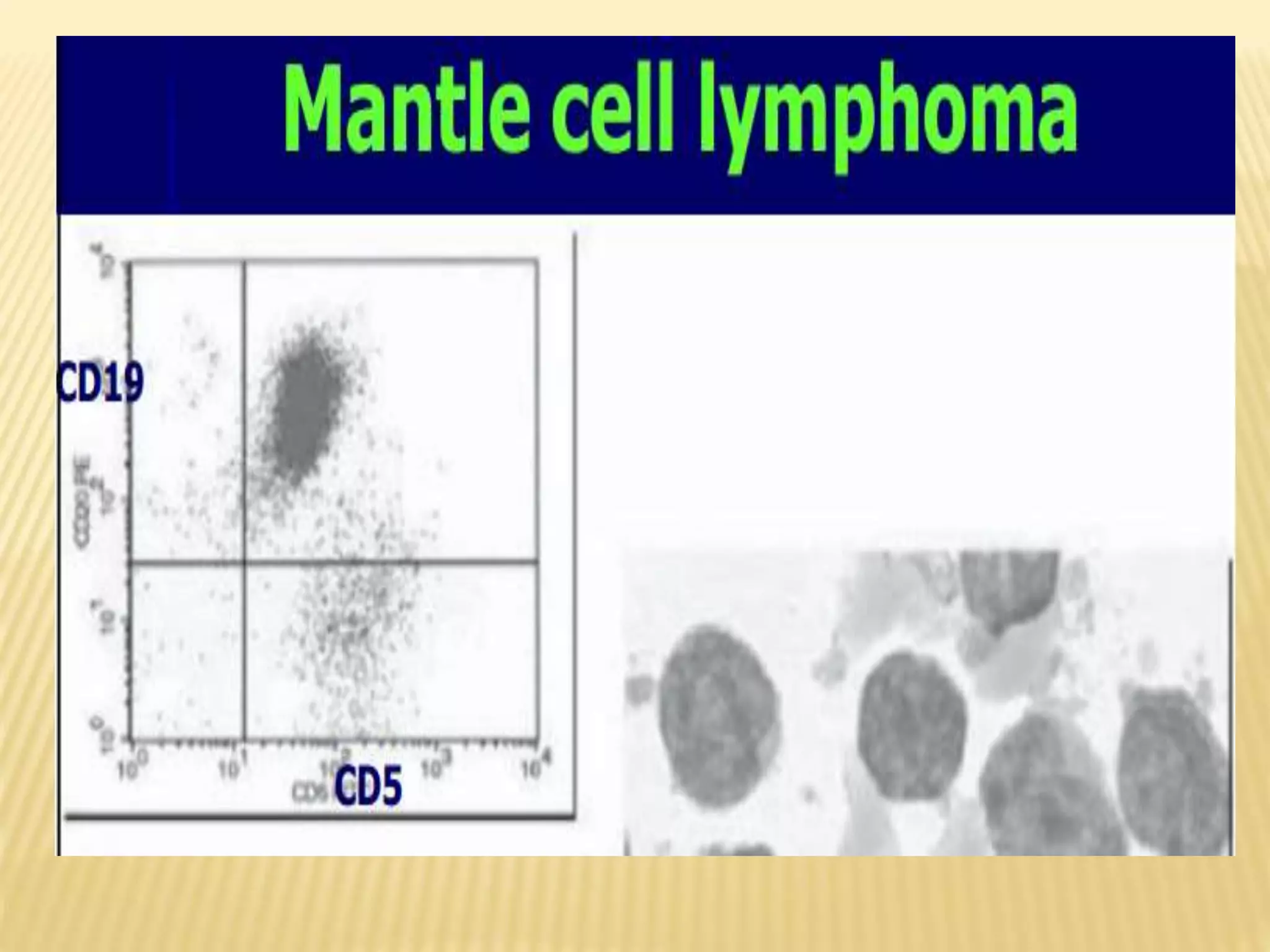
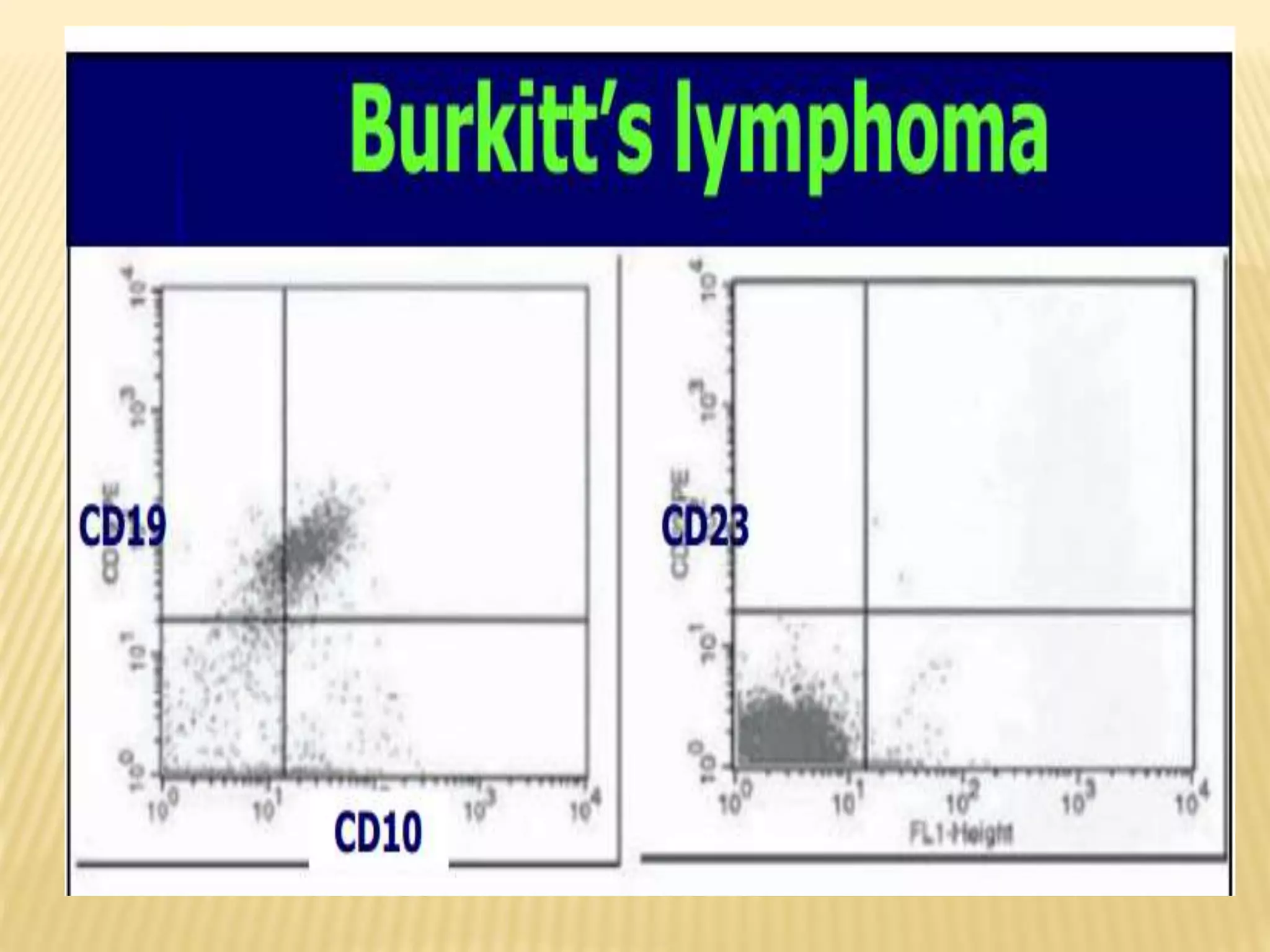
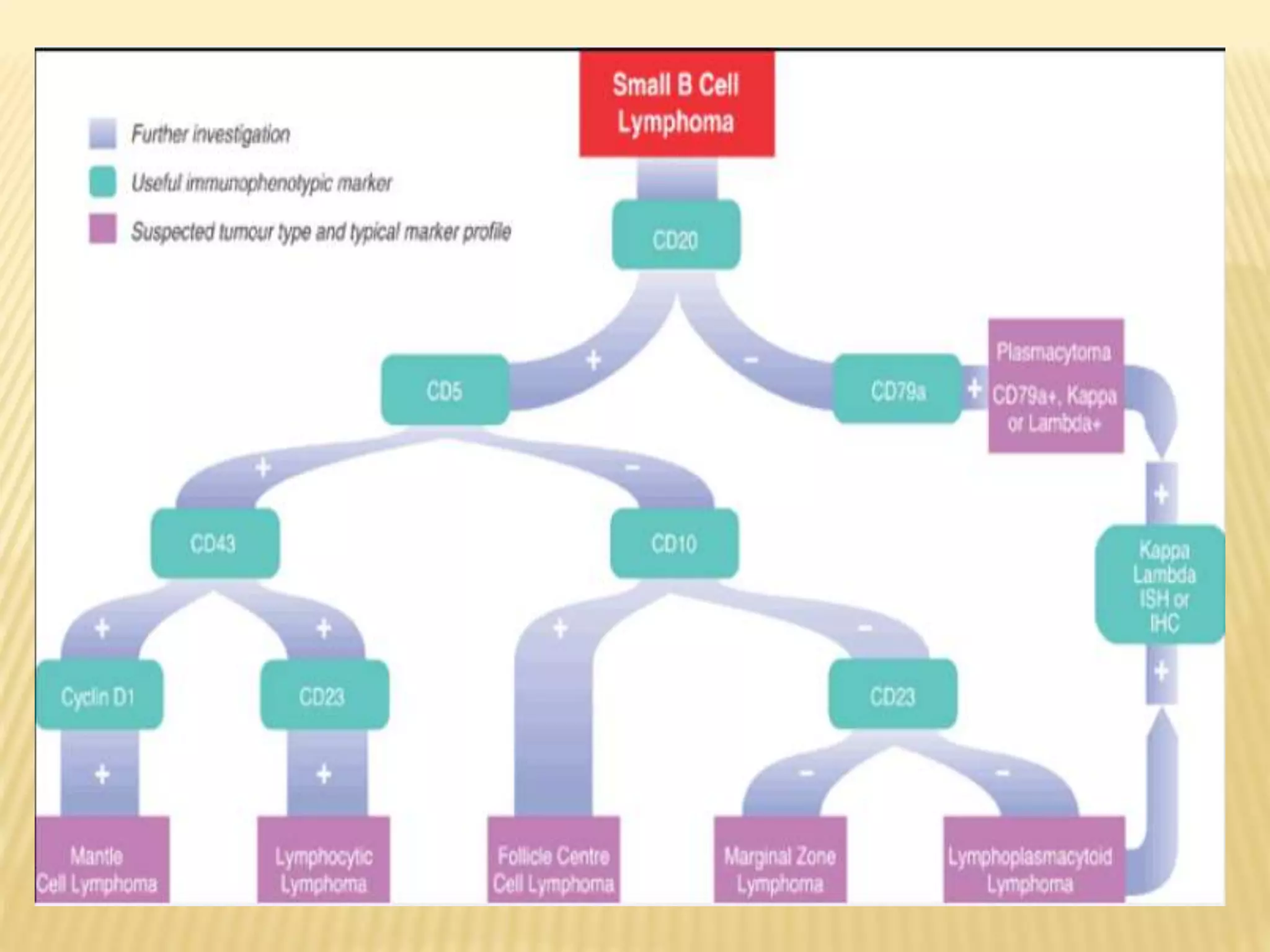
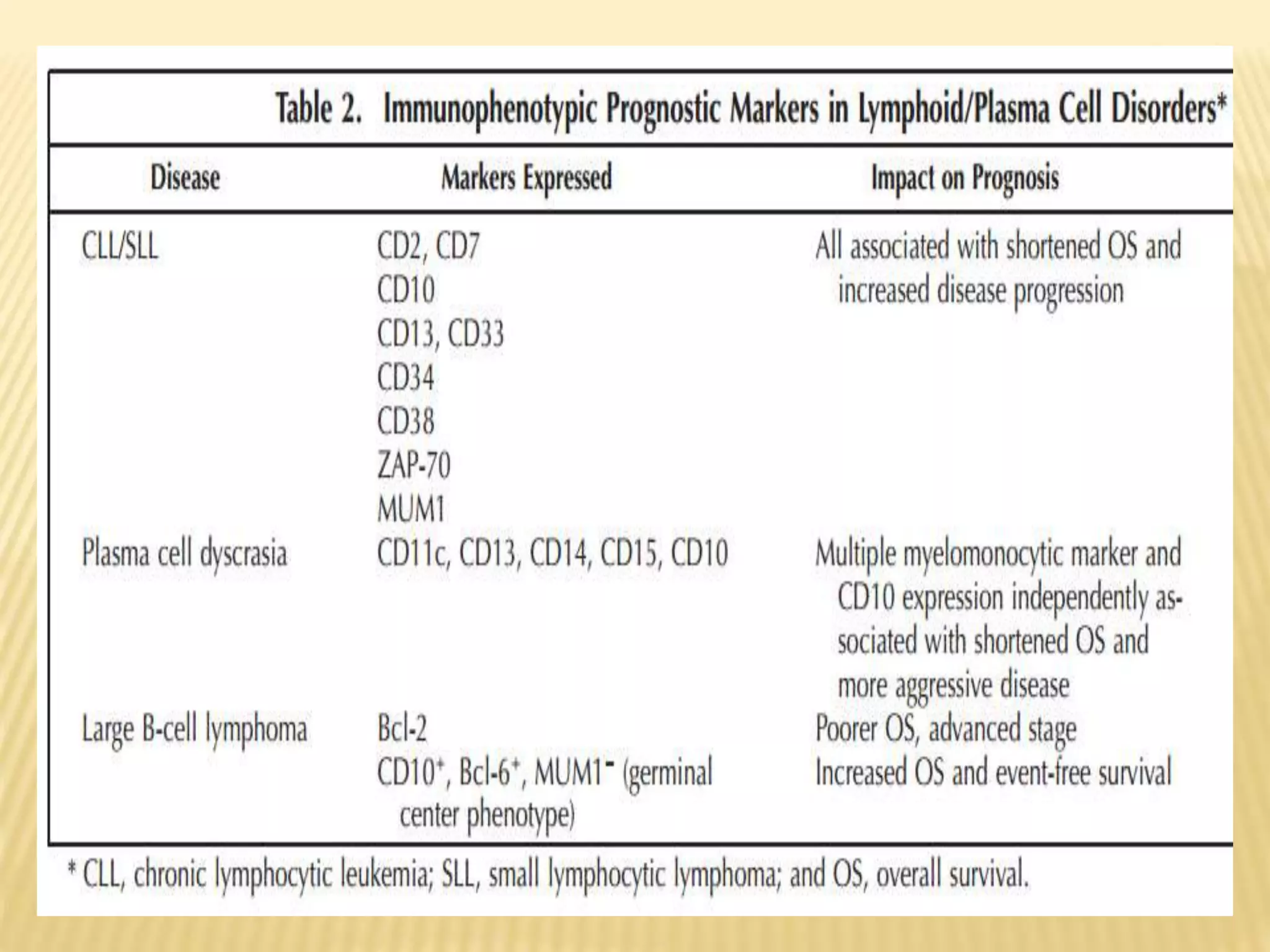
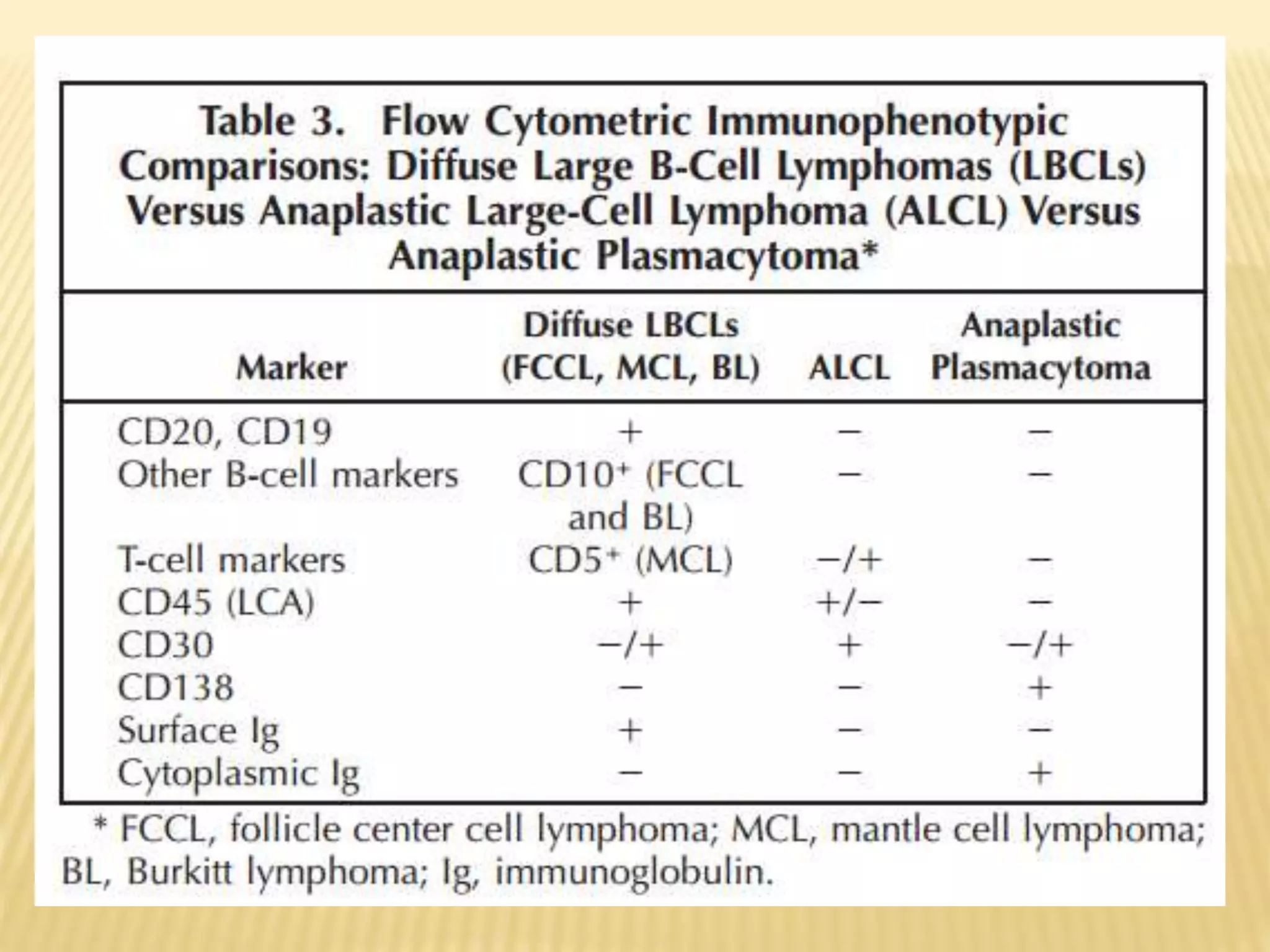
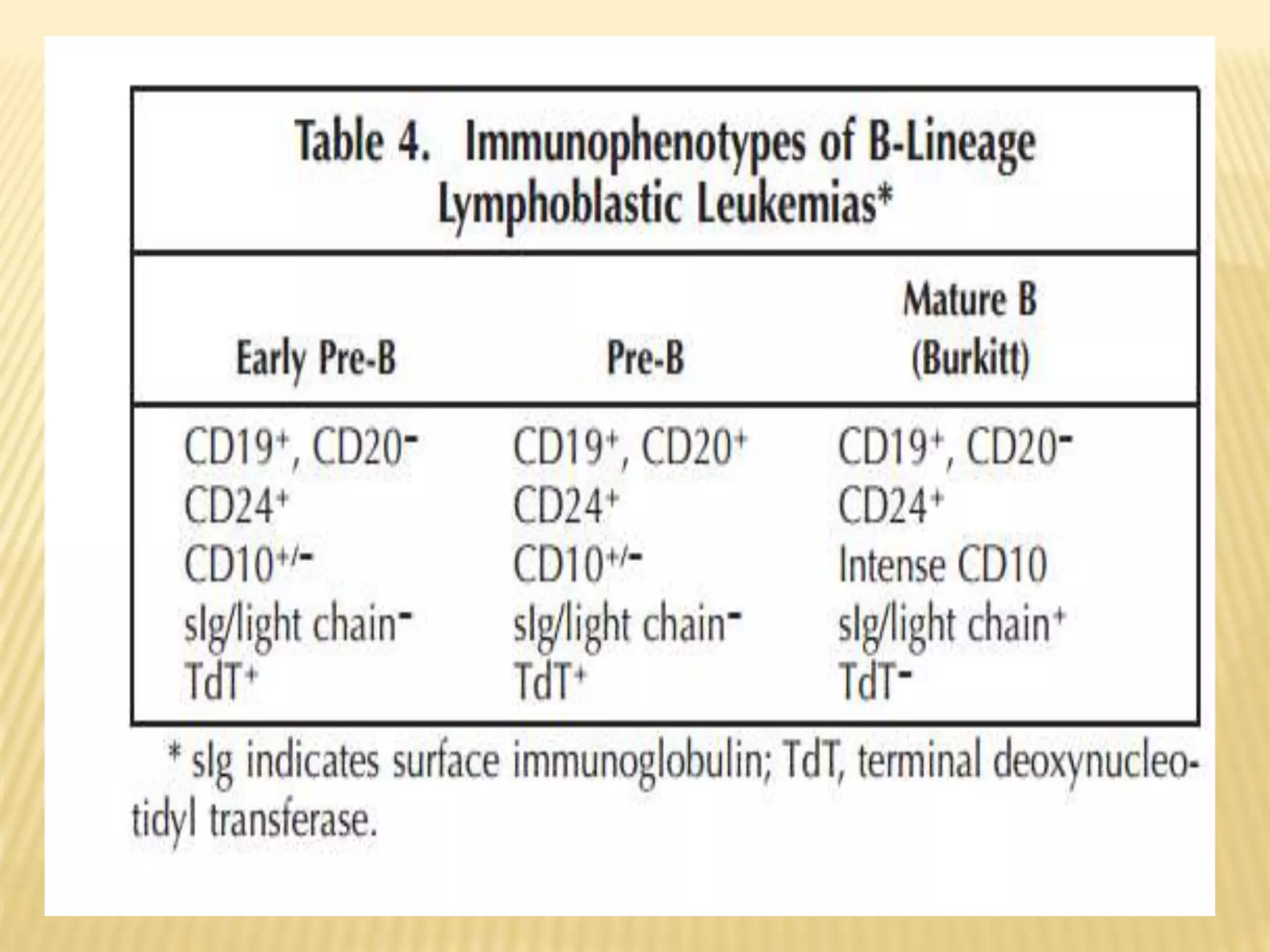
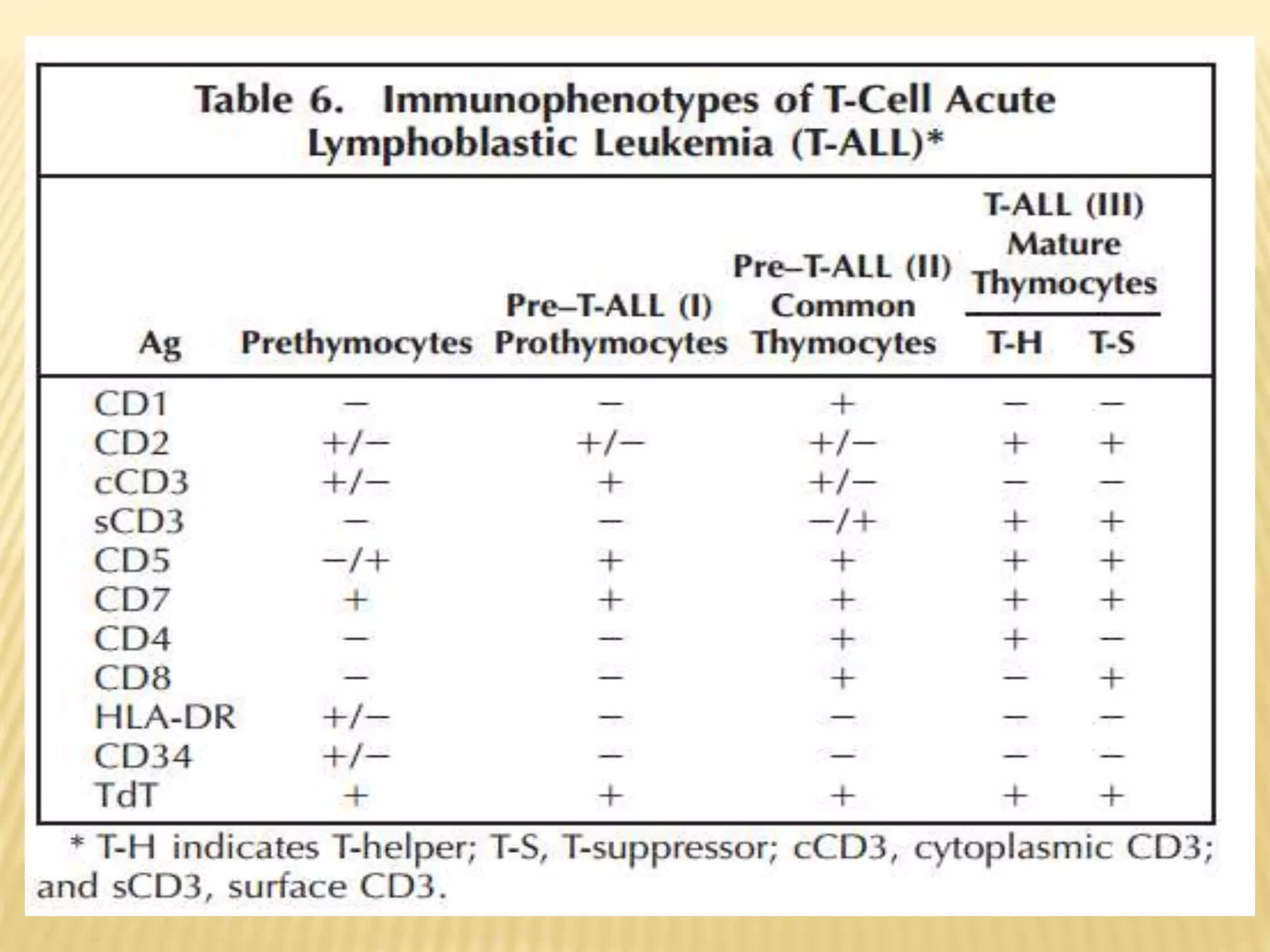
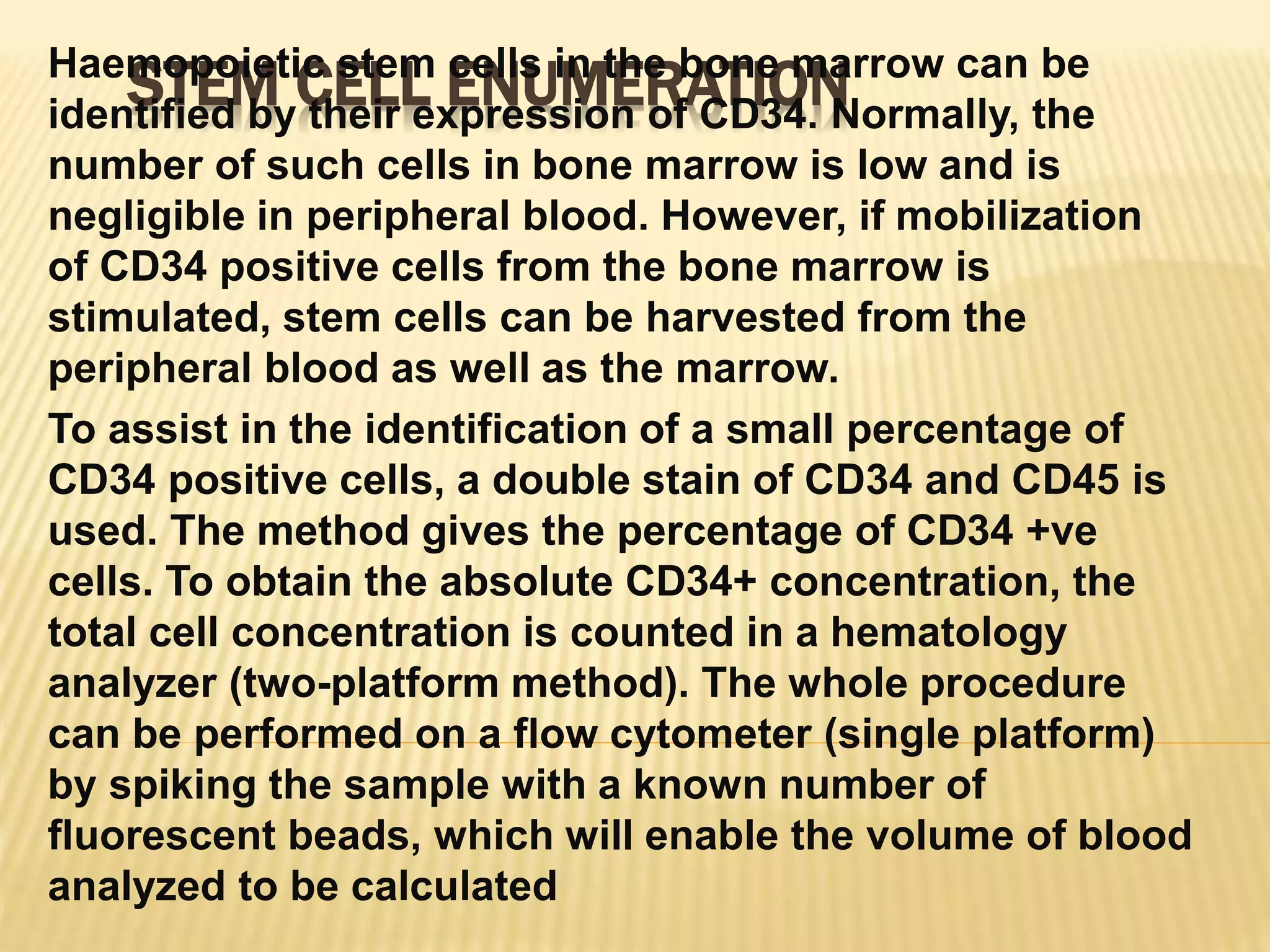
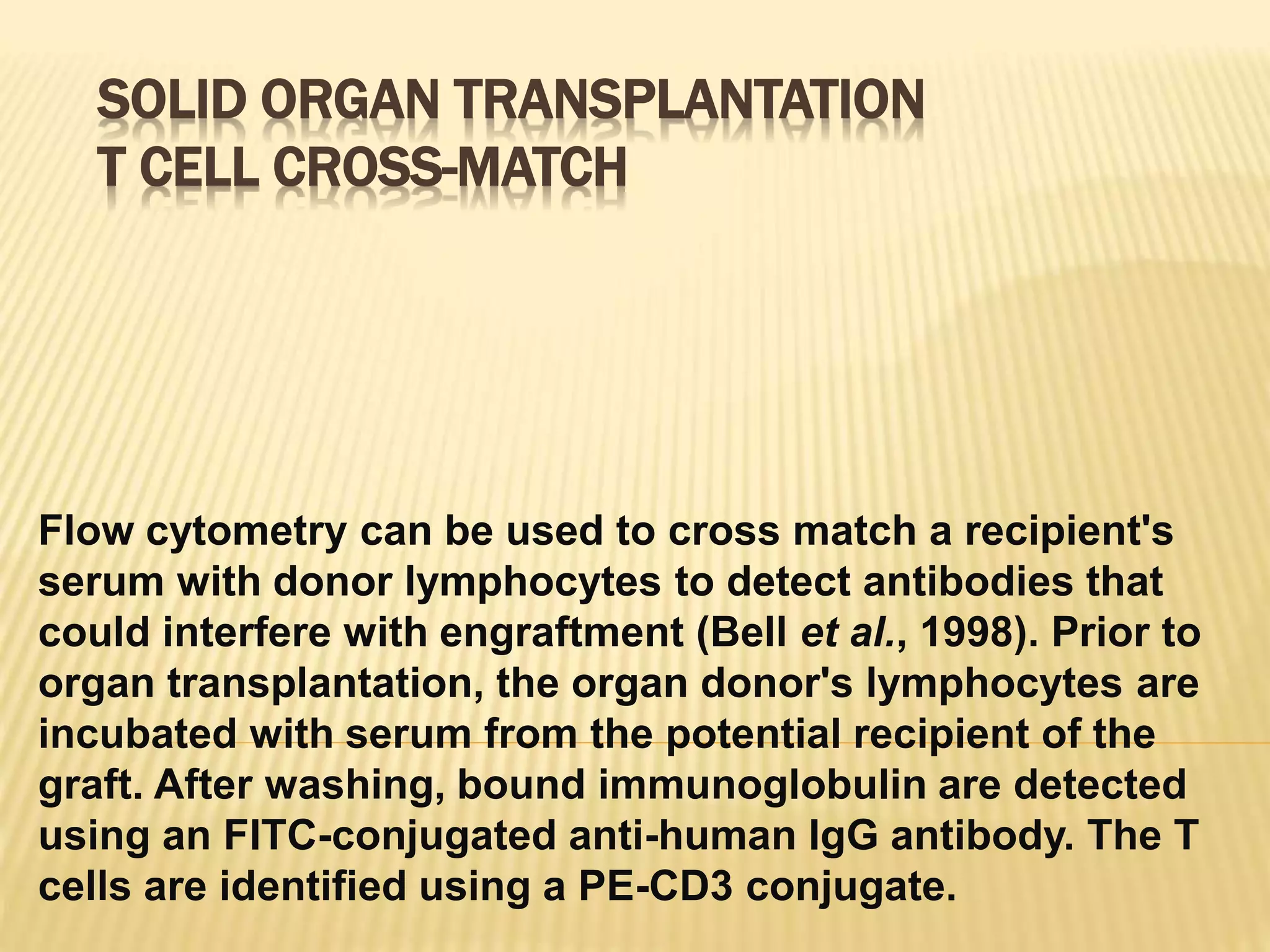
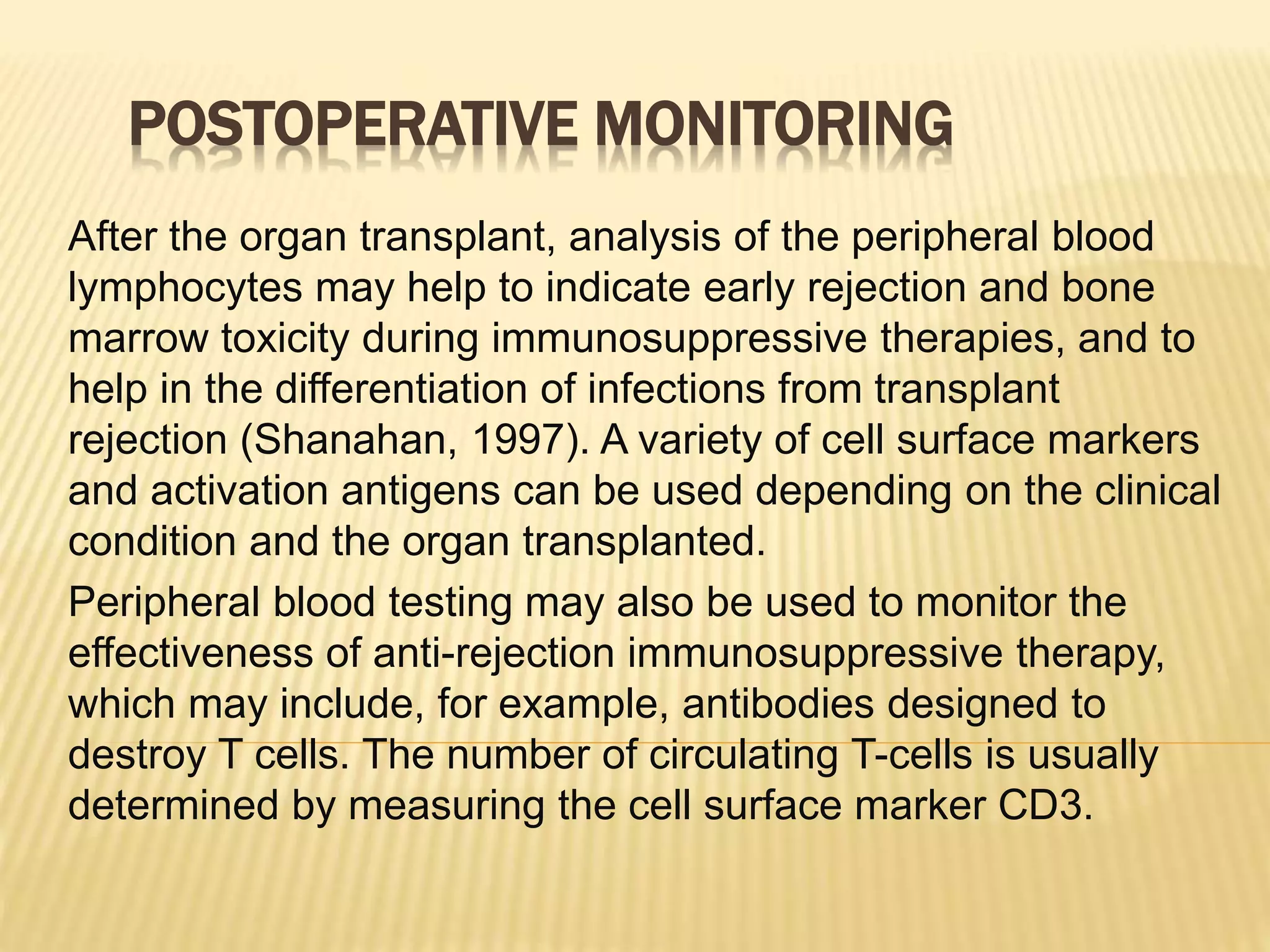
Flow cytometry can be used for a variety of clinical applications including analysis of leukemia and lymphoma through immunophenotyping of cells. It is used to monitor transplant rejection through detection of antibodies and T cell counts. Other uses include stem cell enumeration, detection of autoantibodies, monitoring HIV infection through CD4 counts, analysis of fetal-maternal hemorrhage, evaluation of immunodeficiency diseases, analysis of paroxysmal nocturnal hemoglobinuria, measurement of contaminating leukocytes in blood transfusions, and platelet counting and functional analysis. The principles of flow cytometry involve light scattering and fluorescence detection of cells in a hydrodynamically focused stream that are then separated electrostatically for sorting based on multivariate data analysis.