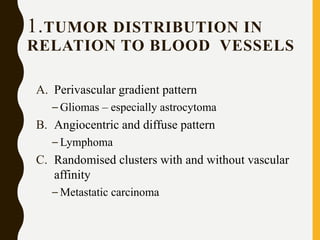
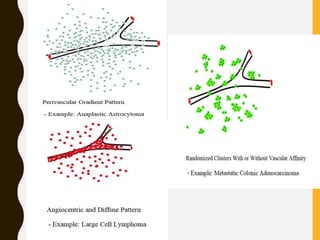
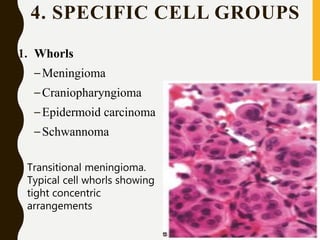
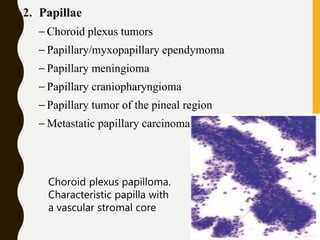
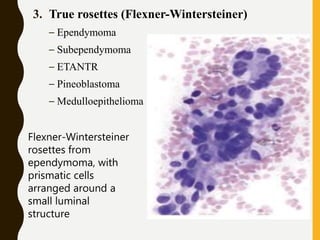
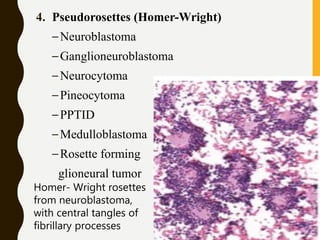
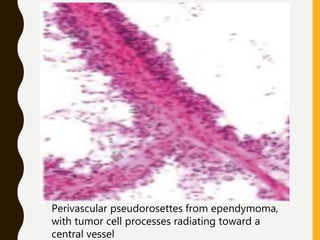
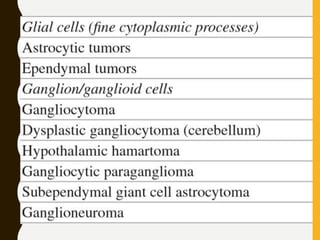
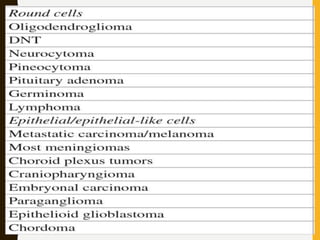
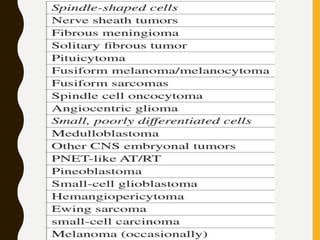
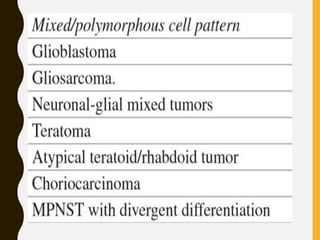
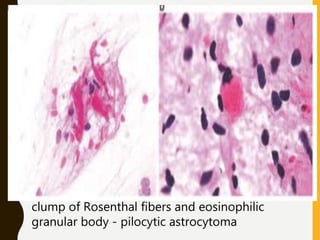
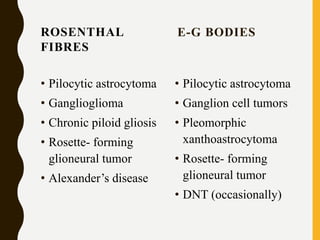
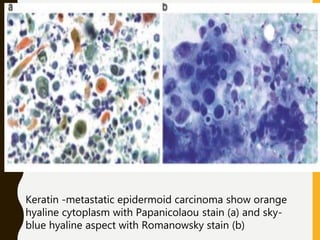
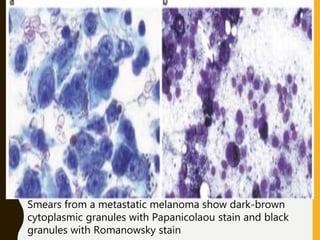
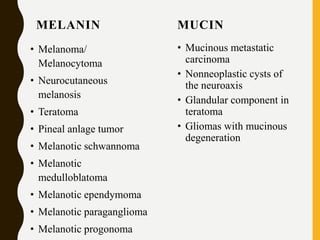
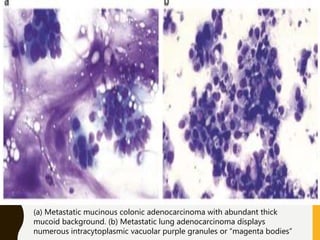
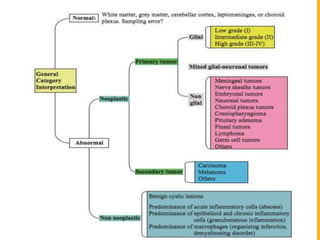
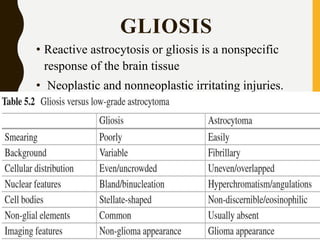
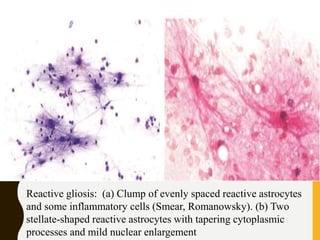
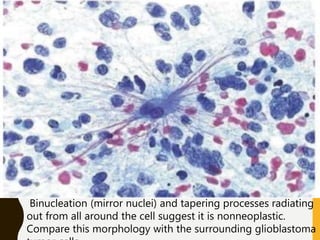
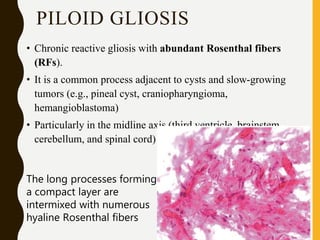
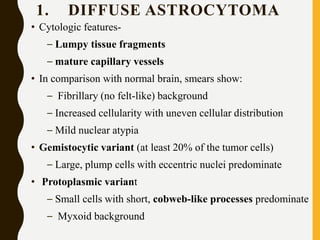
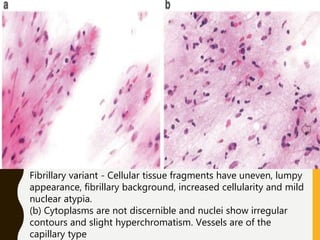
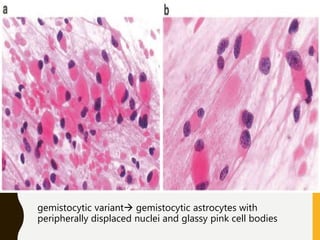
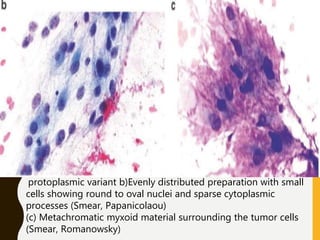
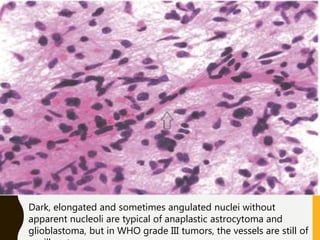
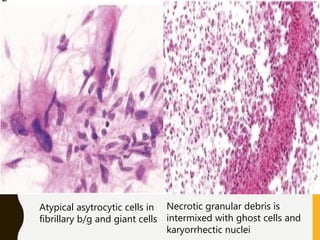
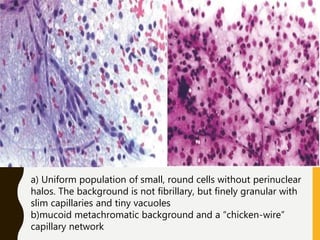
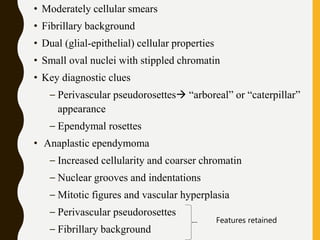
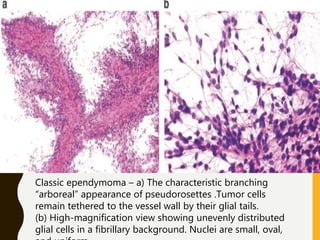
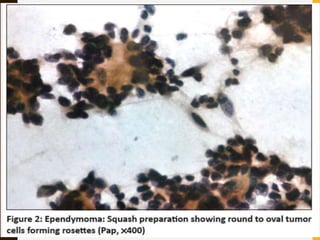
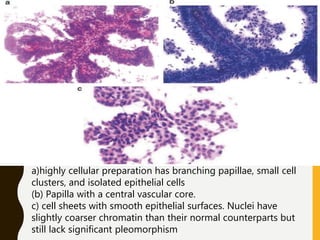
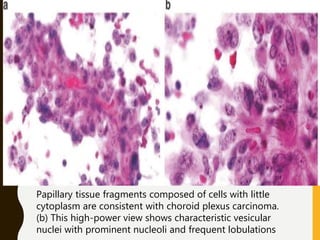
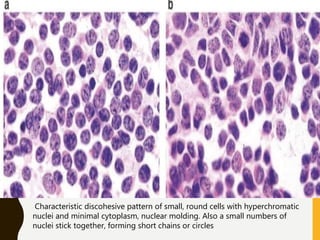
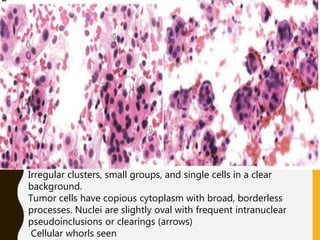
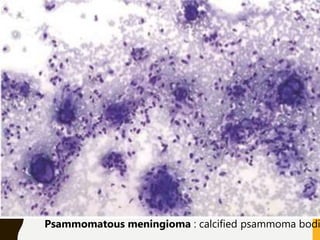
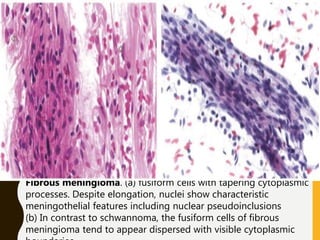
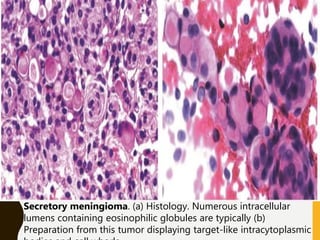
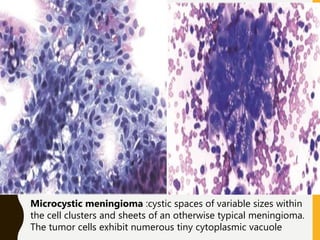
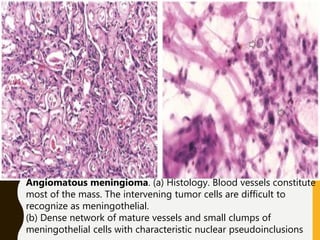
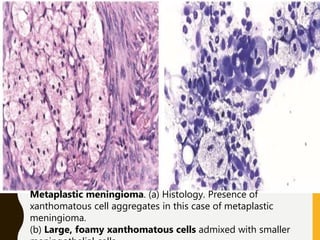
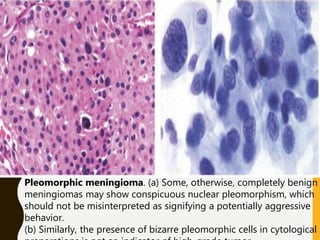
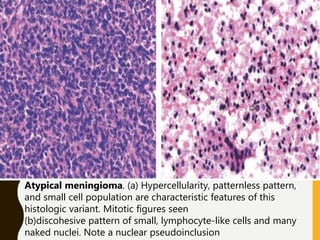
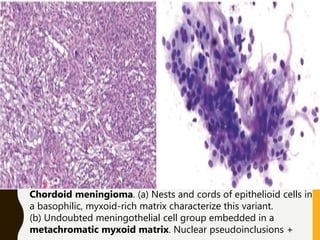
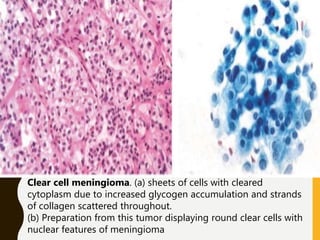
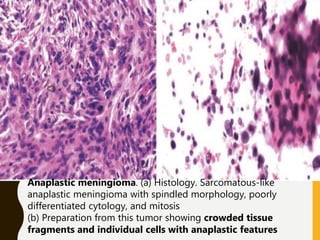
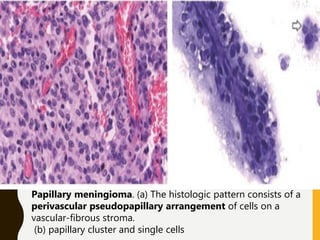
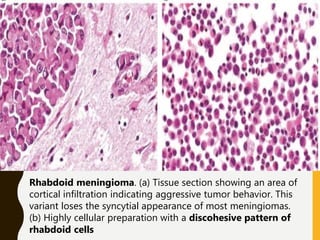
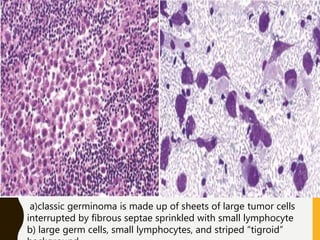
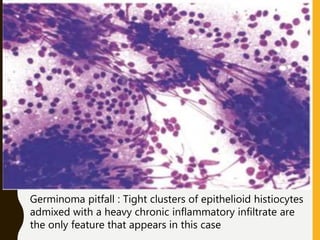
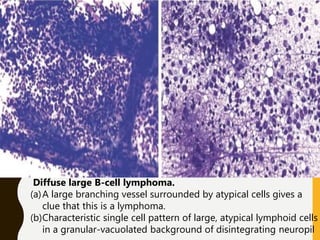
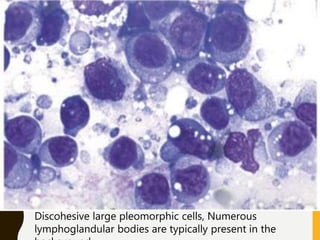
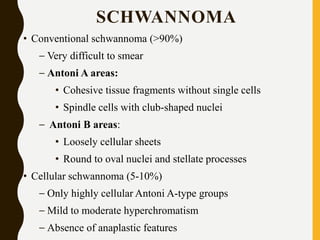
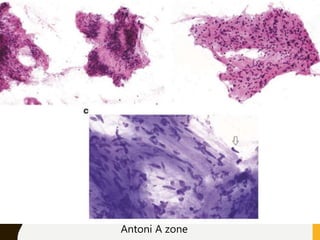
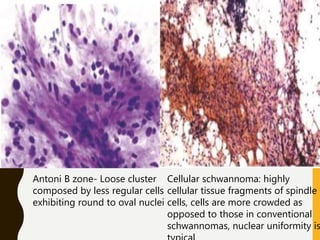
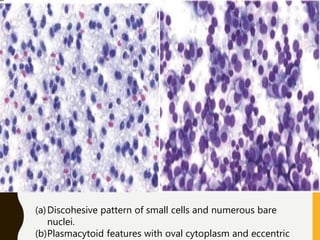
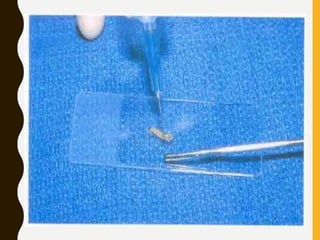
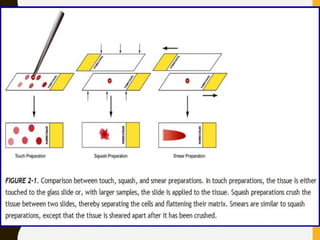
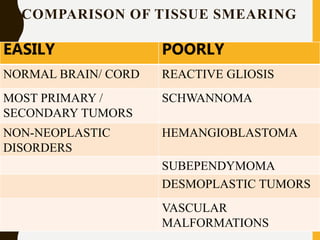
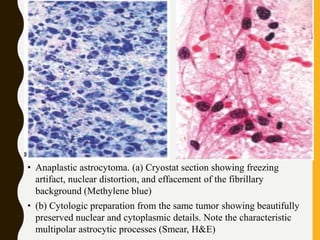
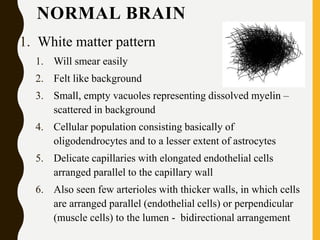
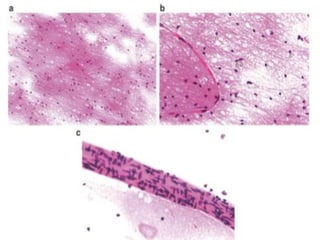
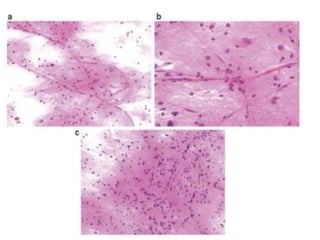
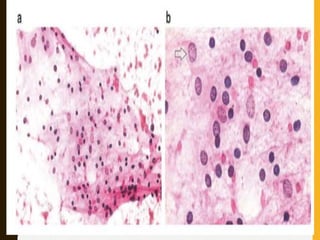
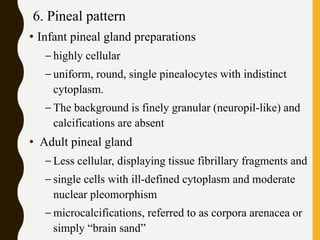
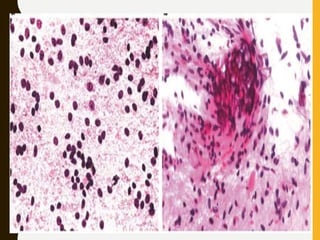
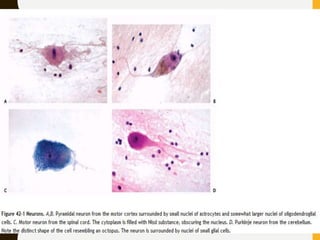
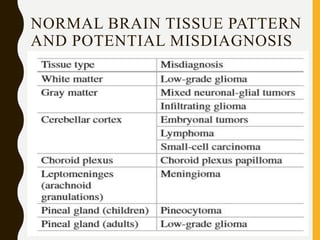
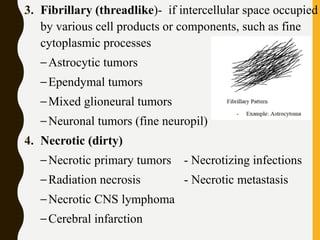
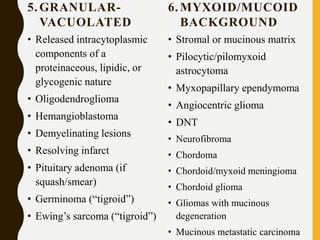
The document discusses the methodology and significance of squash smear cytology in diagnosing central nervous system (CNS) lesions, highlighting its rapid and cost-effective nature. It covers the preparation and interpretation of smears, the types of tissue patterns observed, and specific cellular elements relevant to various CNS tumors. Limitations, advantages, and detailed examples of different cytological patterns are also included, emphasizing the technique's value in intraoperative settings.













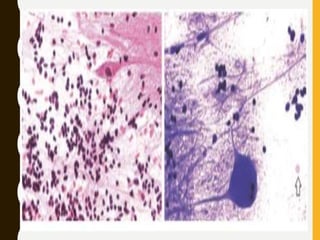
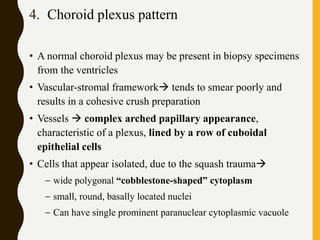
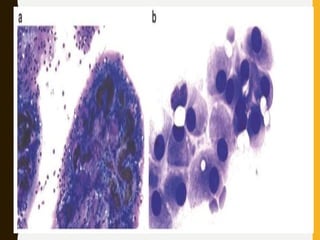
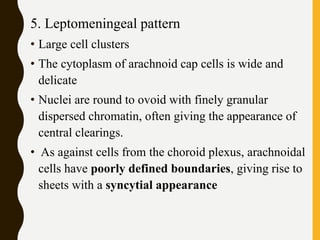
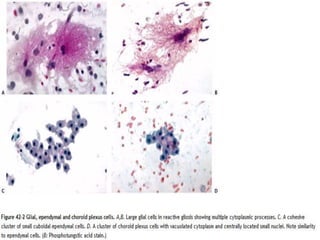











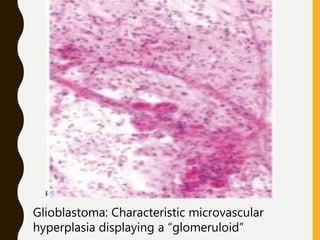
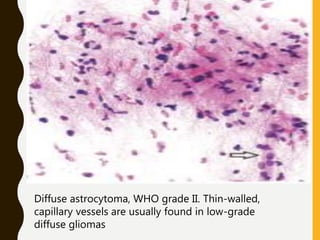
![C. Endothelial cell proliferation (microvascular
hyperplasia/ proliferation [MVP])
–Criterion for anaplasia in diffuse astrocytoma and, to
a lesser degree, in oligodendroglioma and
ependymoma, but not in pilocytic astrocytoma.
–MVP occasionally seen in:
• metastatic cancer (mainly small-cell and renal cell
carcinomas)
• nonneoplastic processes (surrounding resolving
infarctions and abscesses).
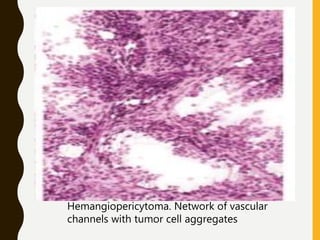
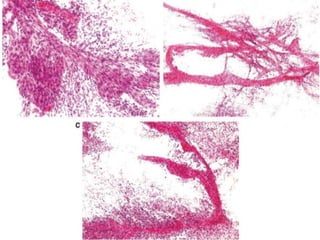
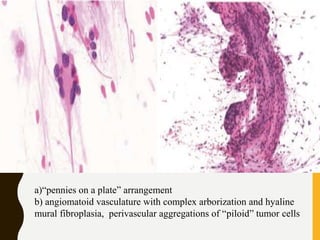
D. Network of vascular channels
– Intricate network of thin-walled vascular channels
characteristic pattern of hemangioblastoma and
hemangiopericytoma.](https://image.slidesharecdn.com/squashsmearcytology-190405132037/85/Squash-smear-cytology-By-Anamika-dev-52-320.jpg)