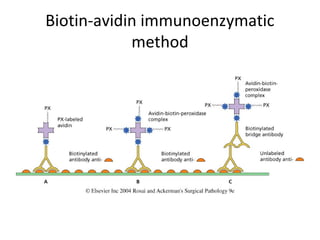
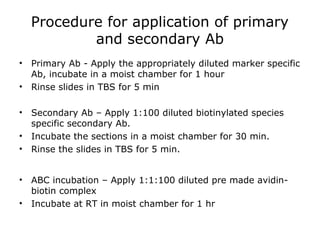
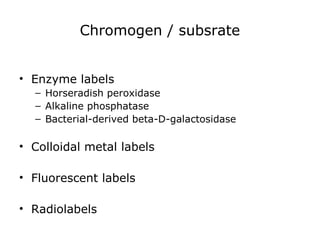
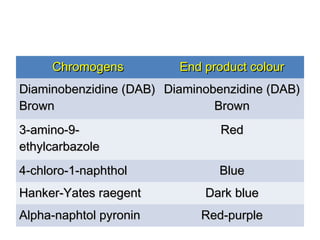
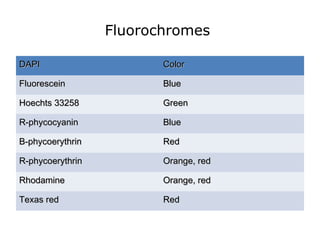
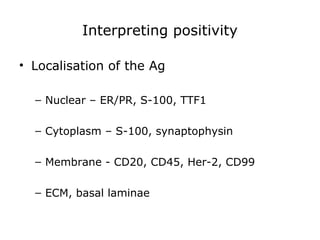
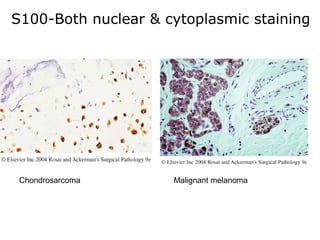
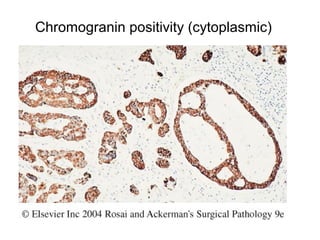
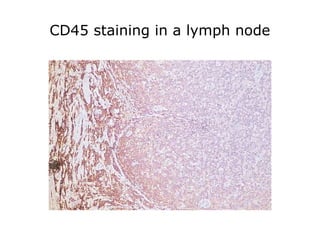
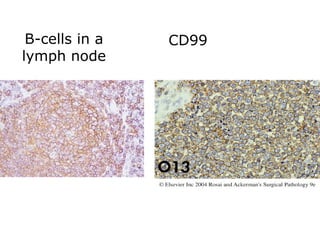
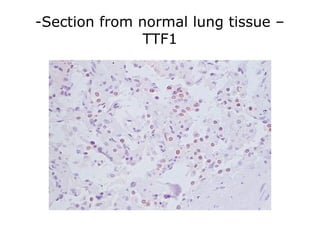
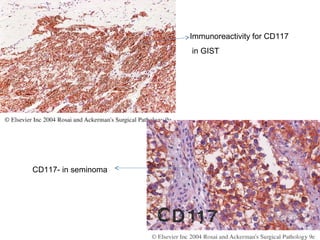
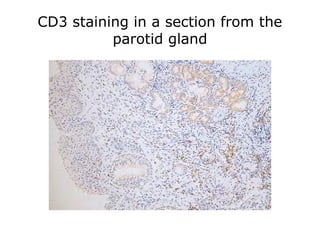
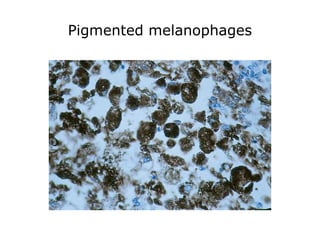
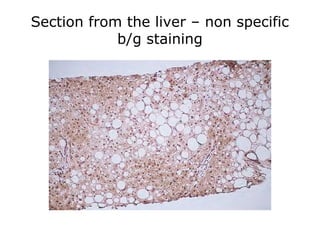
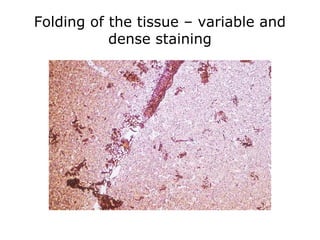
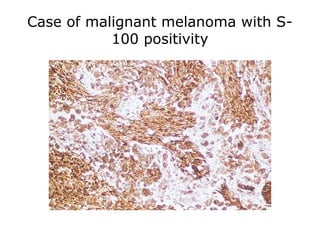
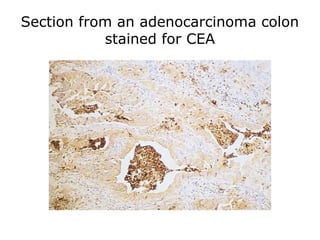
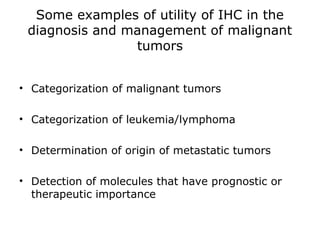
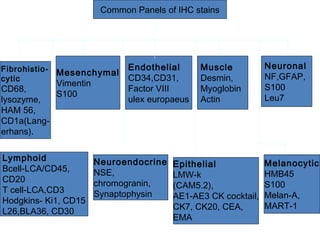
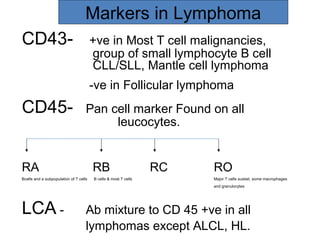
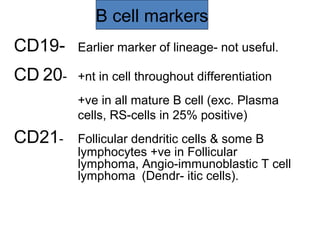
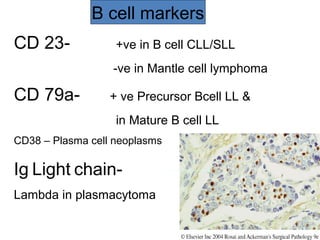
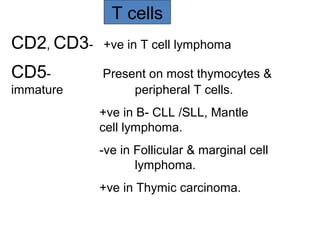
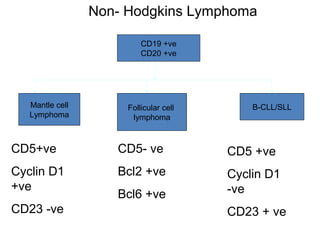
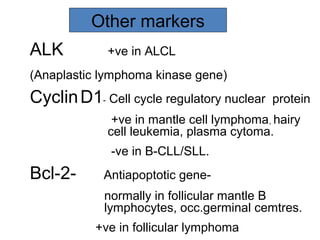
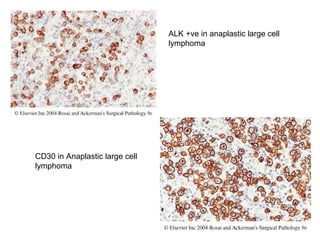
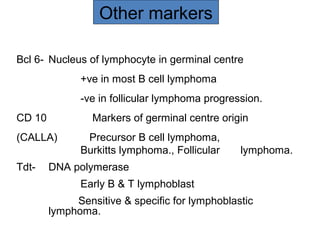
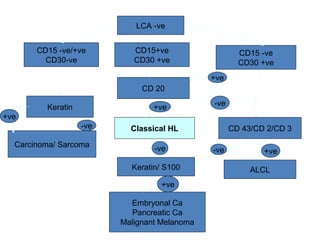
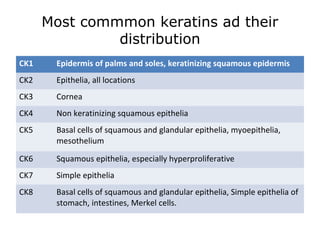
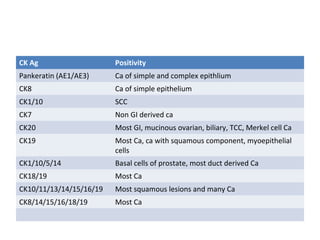
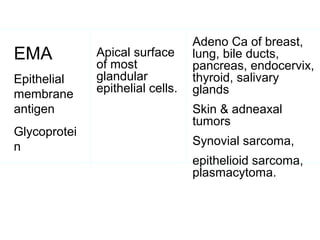
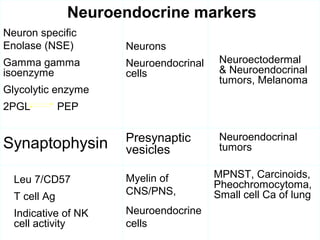
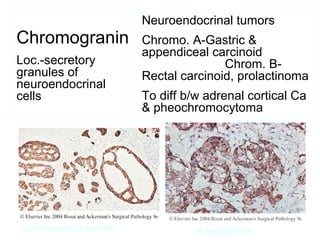
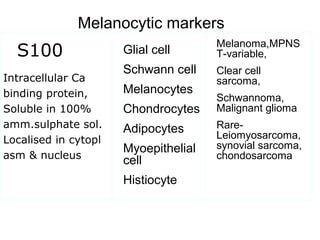
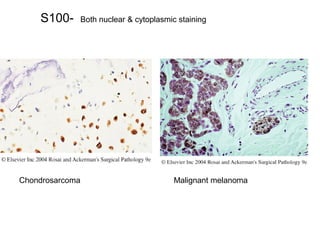
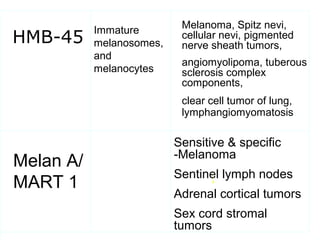
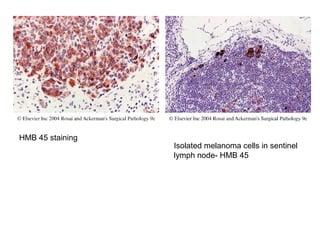
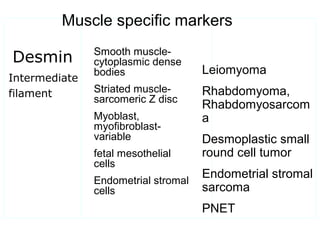
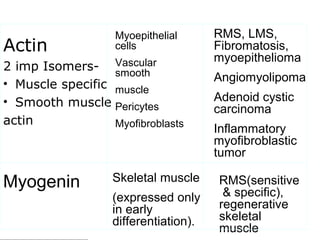
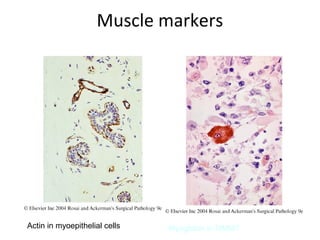
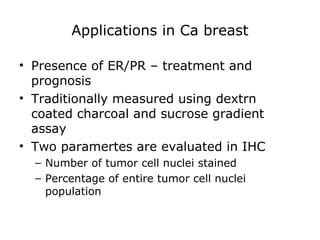
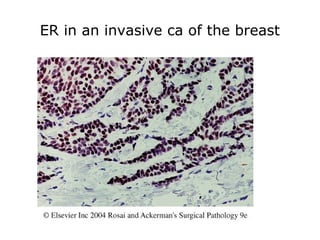
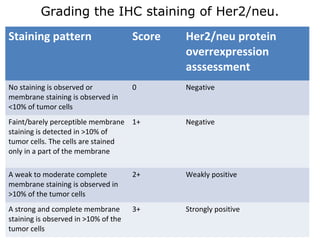
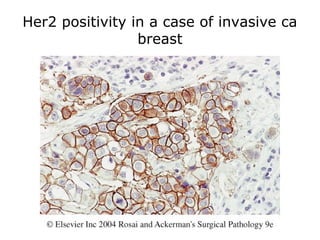
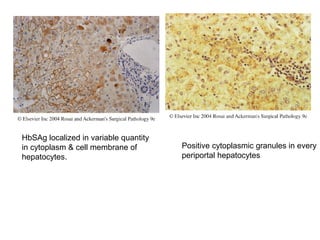
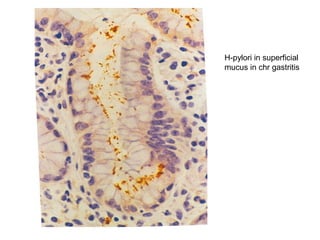
This document provides information on immunohistochemistry (IHC), including:
1. IHC is used to detect antigens in tissues through antigen-antibody recognition at the light microscopic level. It applies immunologic principles and techniques to study cells and tissues.
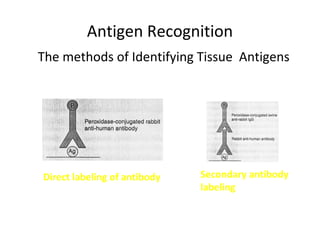
2. The basic principle of IHC is a sharp visualization of target components in cells and tissues based on a satisfactory signal-to-noise ratio.
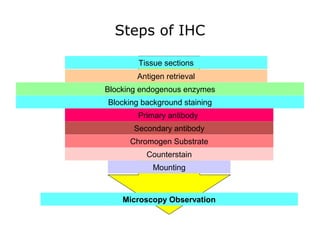
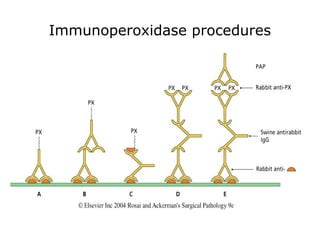
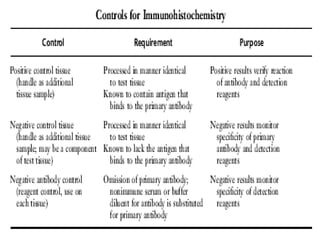
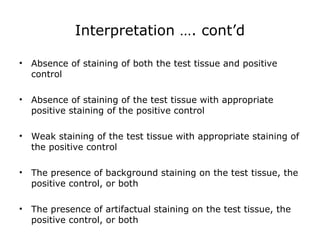
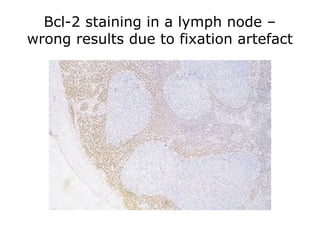
3. The main steps of IHC are tissue processing, antigen retrieval, primary/secondary antibody incubation, detection, counterstaining, and mounting. Proper controls and interpretation of results are also discussed.