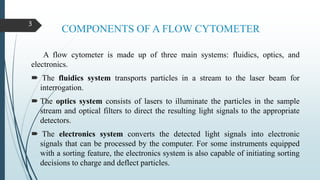
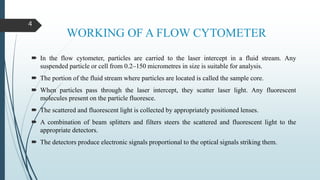
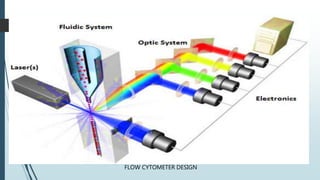
Flow cytometry is a technique that uses lasers to measure physical and chemical properties of cells as they flow in a fluid stream. A flow cytometer consists of three main systems: fluidics to transport cells to the laser, optics including lasers and detectors, and electronics to convert light signals to electronic data. Cells passing through the laser beam scatter and fluoresce light which is detected and used to measure properties like size, granularity, and presence of fluorescent markers. Flow cytometry has many applications in diagnostics including immunophenotyping of leukemias and lymphomas, detecting minimal residual disease, stem cell enumeration, and detecting autoantibodies.