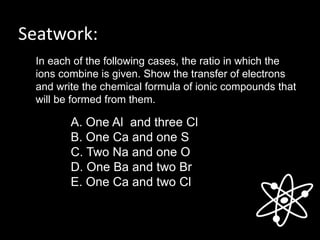
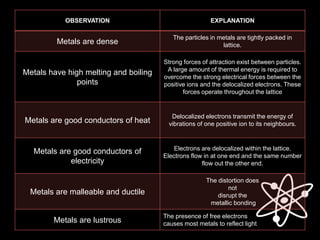
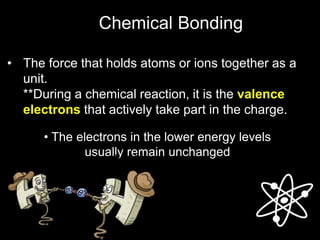
This document discusses different types of chemical bonds:
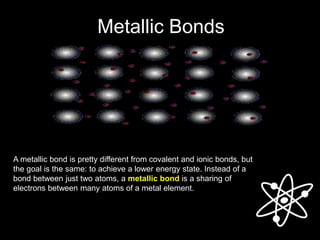
1) Metallic bonds form when valence electrons are delocalized and shared between all metal atoms in a lattice, holding the positive ions together.
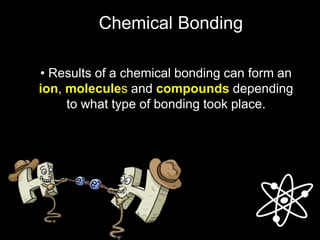
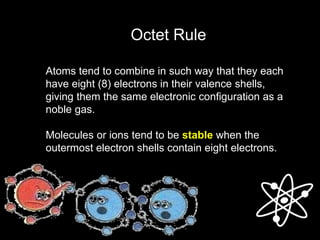
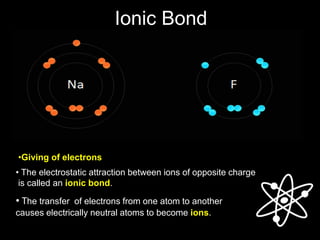
2) Ionic bonds form when a metal transfers electrons to a nonmetal, creating oppositely charged ions that are attracted to each other.
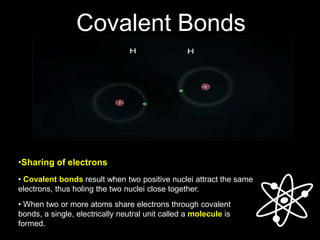
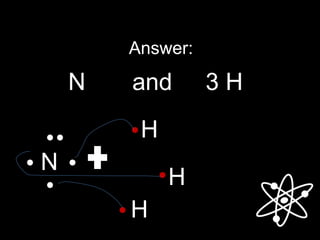
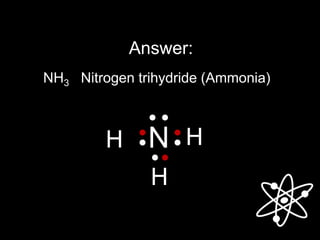
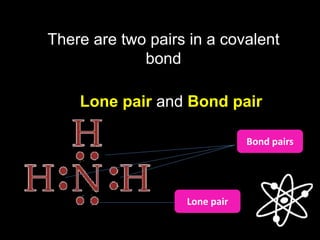
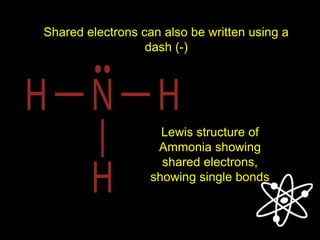
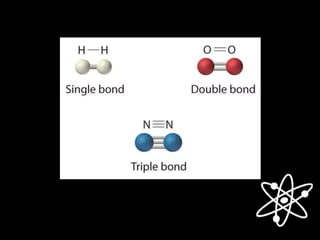
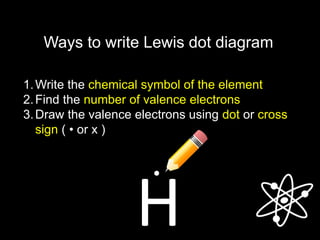
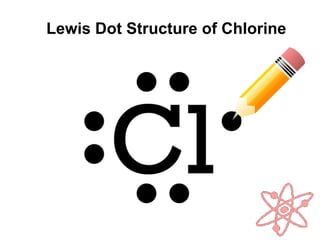
3) Covalent bonds form when two nonmetals share valence electrons in a molecule through electron pairs. Lewis structures are used to represent electron sharing in covalent bonds.





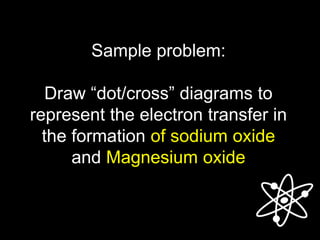
![Answer:
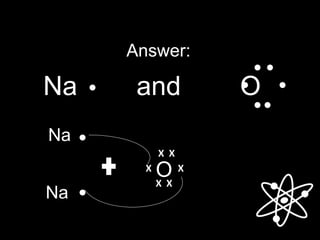
The Lewis structure for Na and O are:
[Na]+
[Na]+
O
X X
X X
X X[ ]
2-
Sodium Oxide (Na2O)](https://image.slidesharecdn.com/chemical-bonding-1-171004101049/85/Chemical-bonding-21-320.jpg)
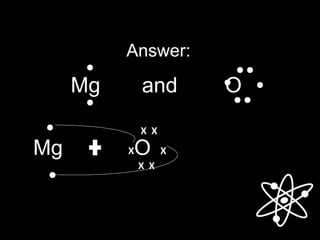
![Answer:
[Mg] O
X X
X X
X X
The Lewis structure for Mg and O are:
[ ]2+ 2-
Magnesium Oxide (MgO)](https://image.slidesharecdn.com/chemical-bonding-1-171004101049/85/Chemical-bonding-23-320.jpg)