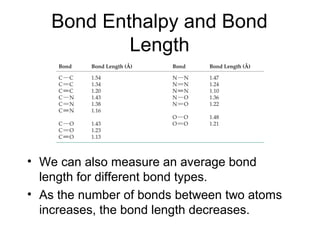
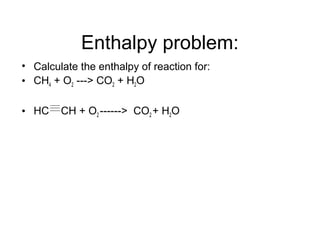
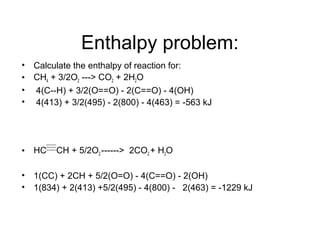
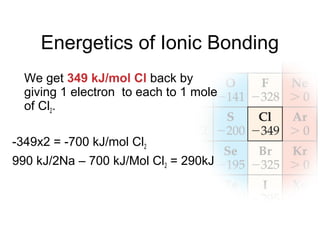
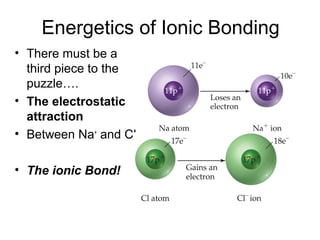
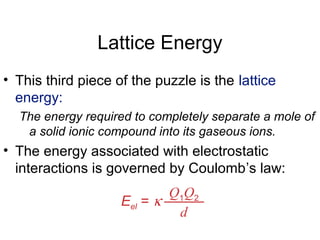
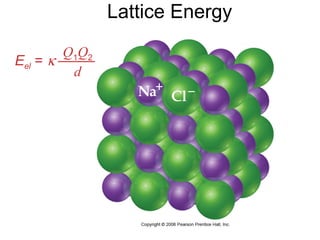
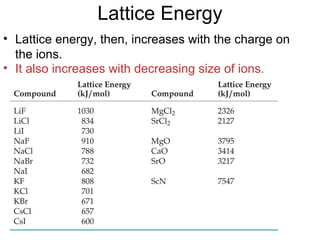
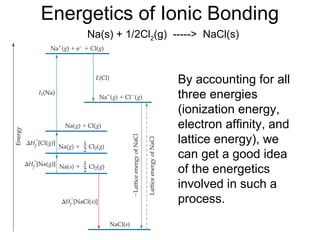
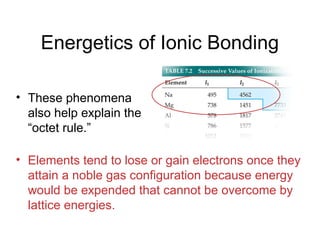
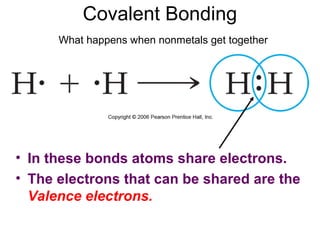
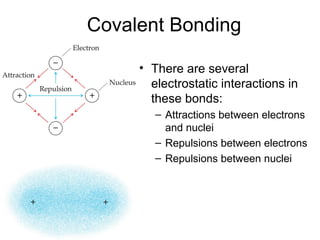
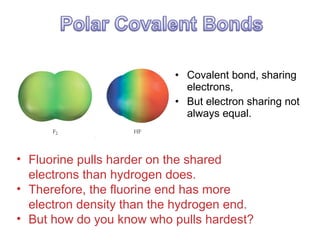
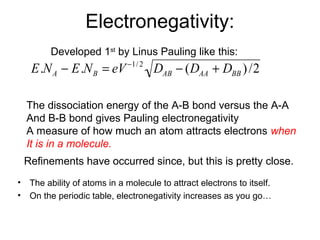
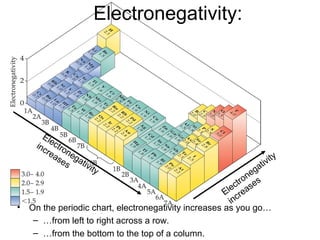
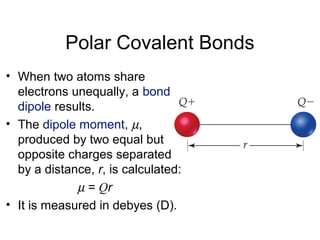
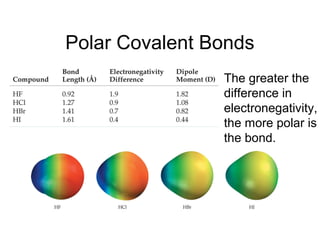
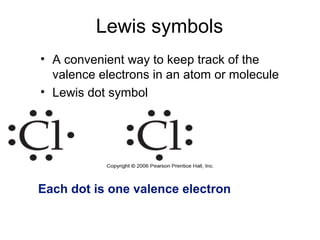
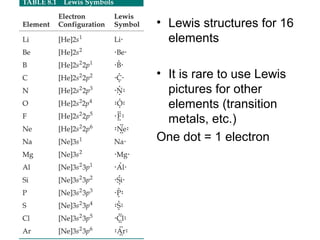
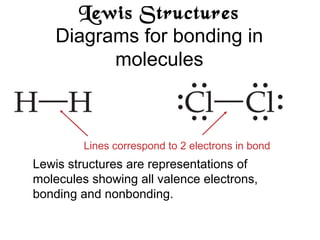
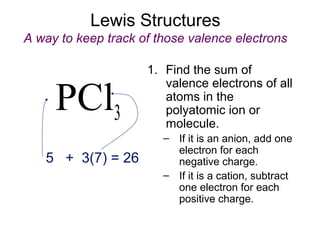
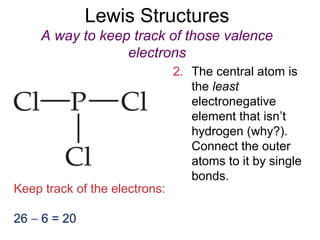
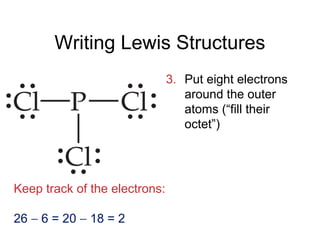
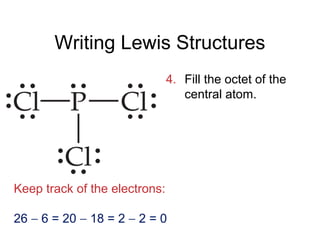
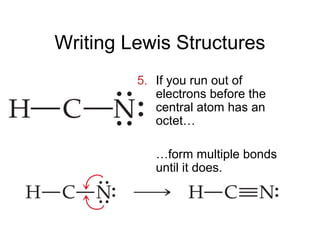
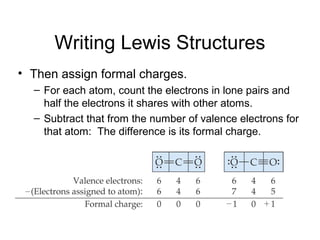
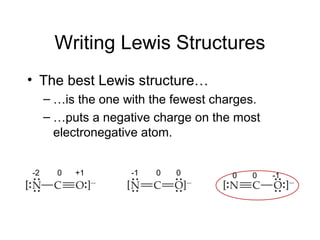
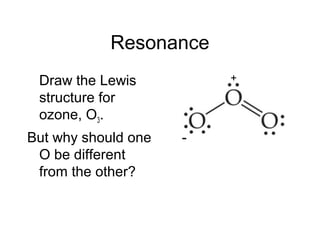
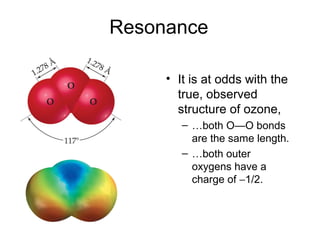
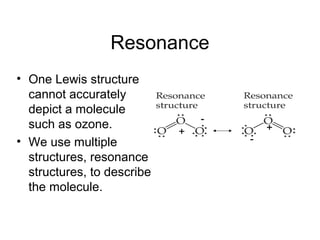
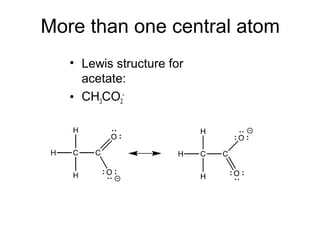
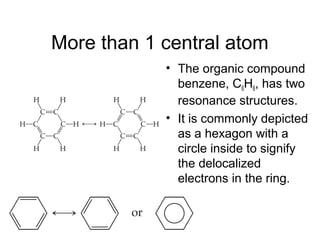
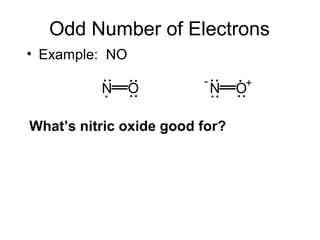
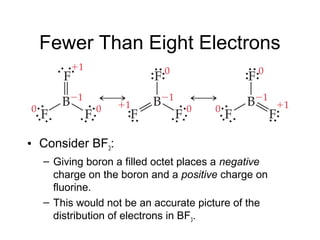
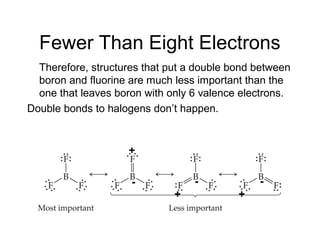
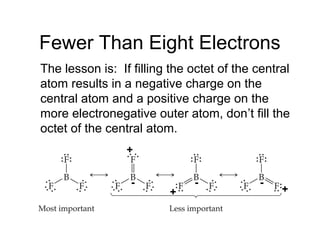
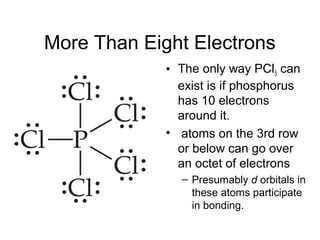
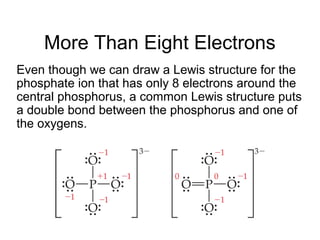
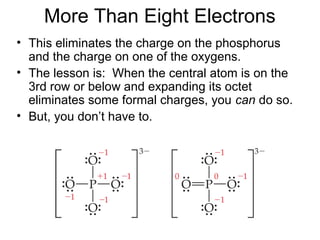
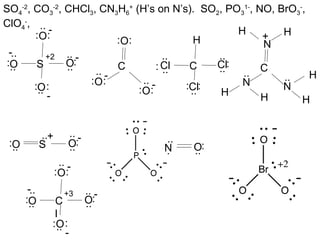
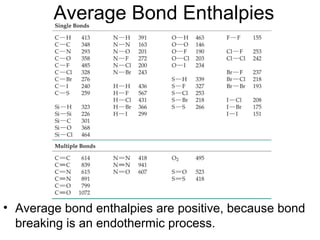
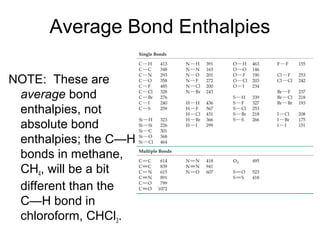
This document discusses different types of chemical bonds including ionic, covalent, and metallic bonds. It describes the formation of ionic bonds between metals and nonmetals and how ionization energy, electron affinity, and lattice energy contribute to the energetics of ionic bonding. Covalent bonding is explained as the sharing of electrons between nonmetals. Factors that determine bond polarity like electronegativity are also covered. The document provides details on writing Lewis structures, accounting for valence electrons and formal charges. Exceptions to the octet rule for molecules with odd numbers of electrons, incomplete octets, and expanded octets are explained.



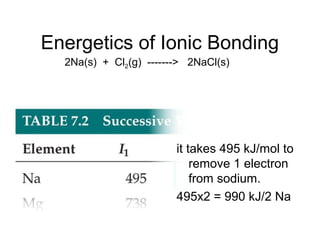












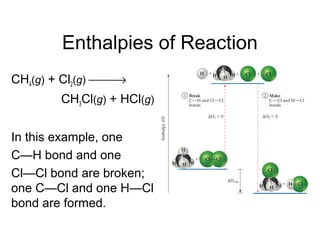
![Enthalpies of Reaction
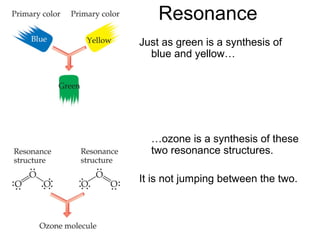
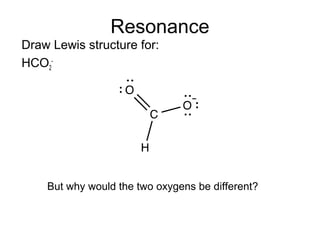
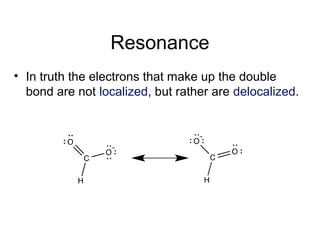
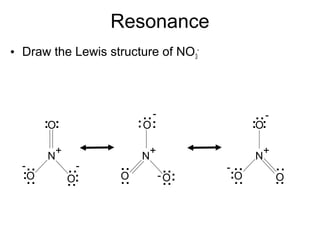
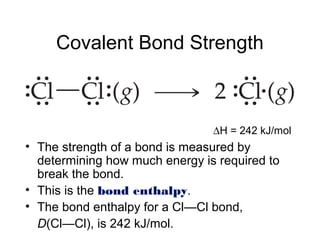
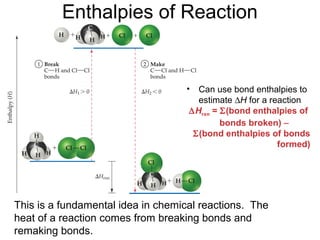
So,
∆Hrxn = [D(C—H) + D(Cl—Cl) − [D(C—Cl) + D(H—Cl)
= [(413 kJ) + (242 kJ)] − [(328 kJ) + (431 kJ)]
= (655 kJ) − (759 kJ)
= −104 kJ
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)](https://image.slidesharecdn.com/chap8lect2015-180727085904/85/Chap1-66-320.jpg)