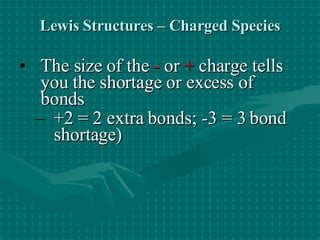
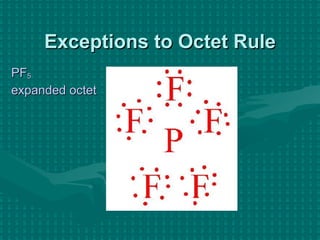
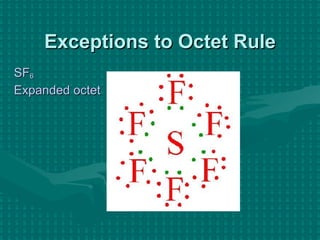
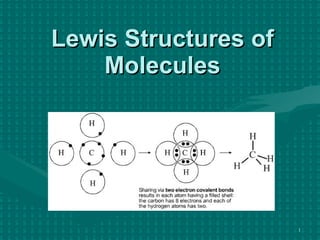
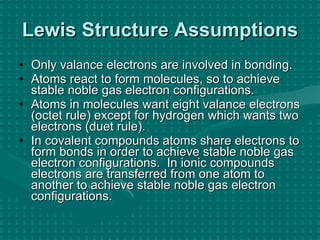
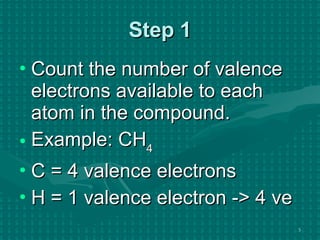
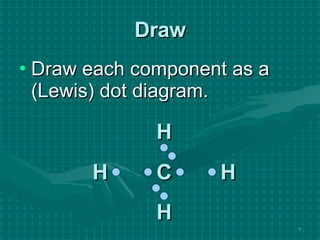
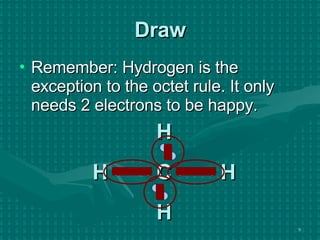
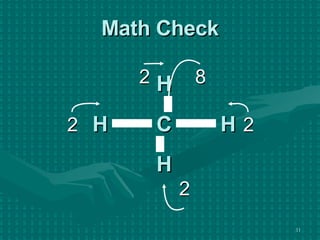
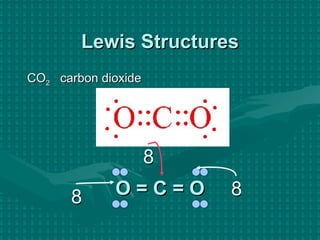
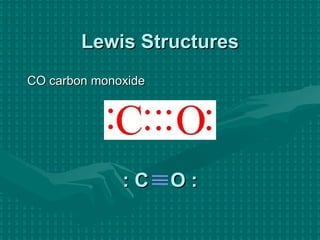
The document discusses Lewis structures and the rules for drawing them. It explains that Lewis structures show how atoms bond via shared electron pairs to achieve stable noble gas configurations. It provides a 4-step process for drawing Lewis structures, covering counting electrons, identifying the central atom, adding lone pairs to complete octets, and checking that all electrons are accounted for. Exceptions to the octet rule and drawing structures for ions are also covered.






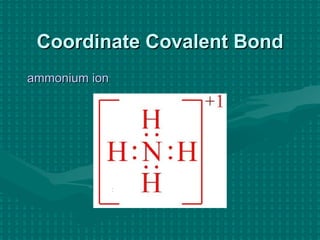
![Rules for Molecules with an Overall Charge When figuring out the number of electrons available, make sure to add or subtract as indicated by the charge. Create the Lewis structure the same as always. Put square brackets [ ] around the structure. Write the charge in a superscript. Called a “Coordinate Covalent Bond”](https://image.slidesharecdn.com/lewis-structures4662/85/Lewis-Structures-16-320.jpg)