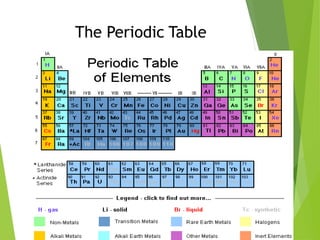
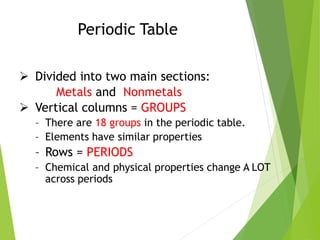
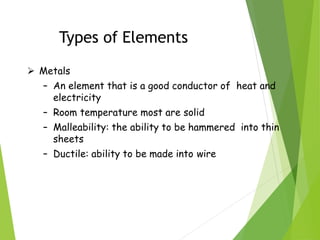
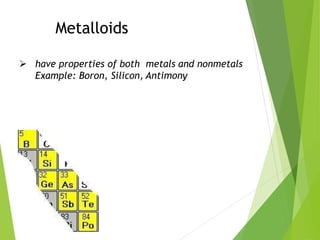
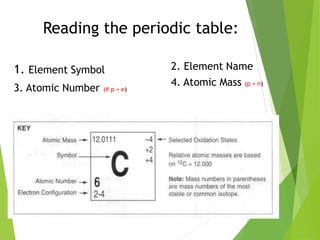
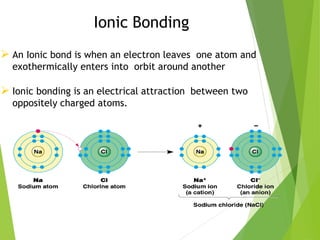
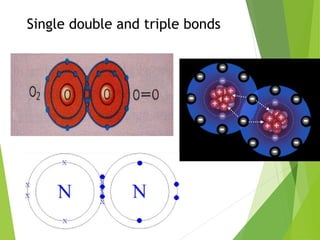
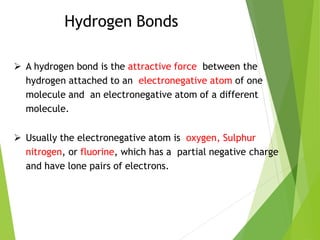
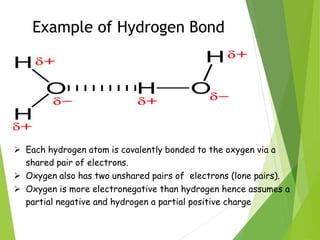
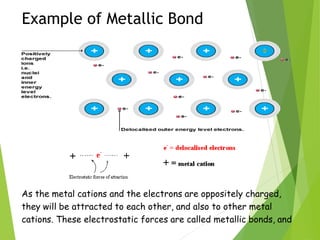
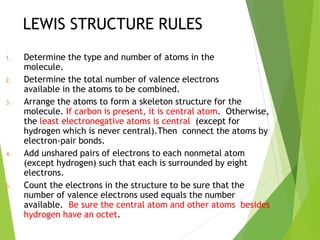
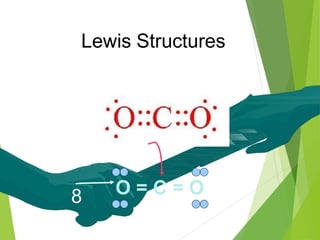
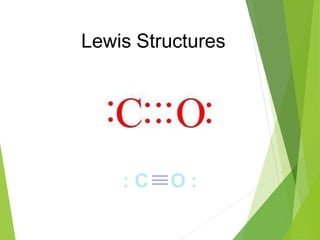
The document provides an overview of the periodic table, detailing its organization into metals, nonmetals, and various groups like alkali metals and transition metals. It also covers the types of chemical bonding, including ionic, covalent, hydrogen, and metallic bonds, emphasizing the principles of electron transfer and sharing in bond formation. Additionally, it introduces Lewis structures as a method for representing covalent bonds and outlines their application in understanding molecular stability.