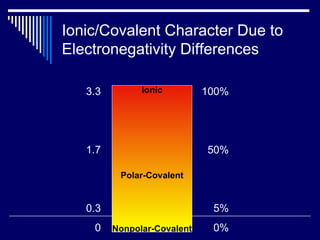
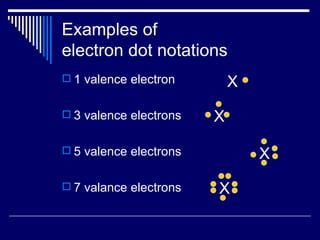
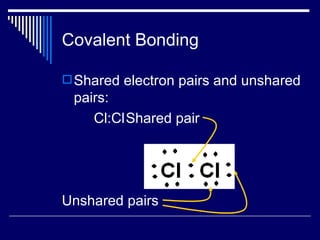
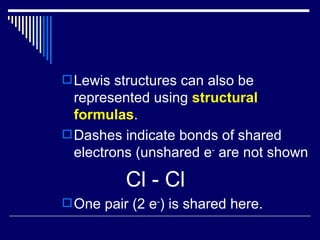
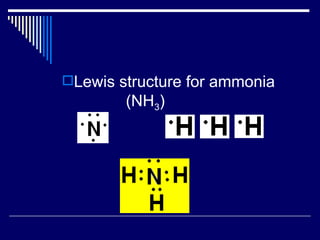
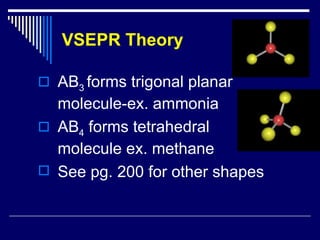
Chemical bonds form between atoms to achieve more stable arrangements with lower potential energy. The type of bonding depends on differences in electronegativity between atoms. Ionic bonds form between ions, covalent bonds involve shared electron pairs, and metallic bonds result from delocalized electrons shared among many atoms in a lattice. Molecular geometry and intermolecular forces also influence molecular properties.