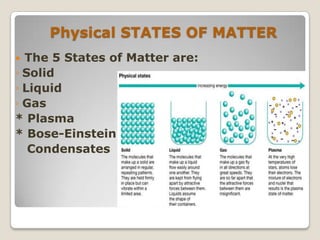
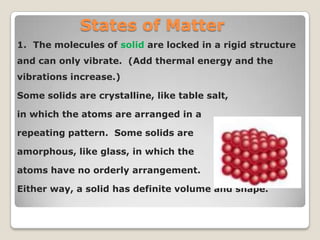
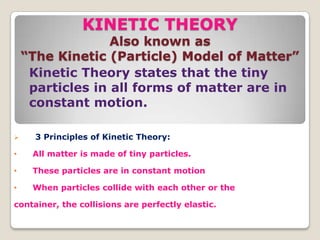
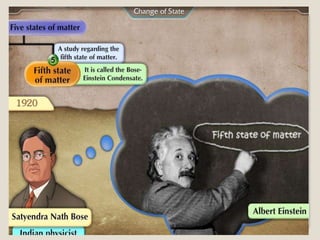
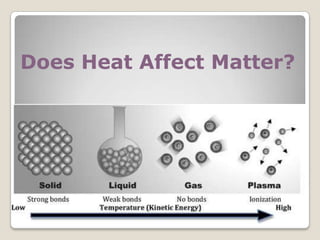
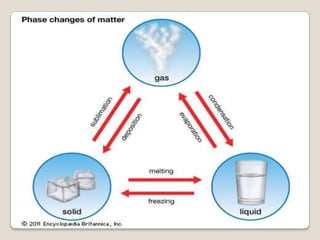
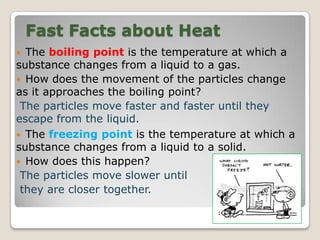
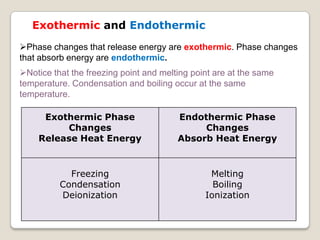
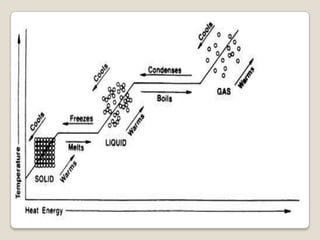
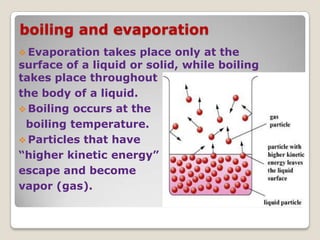
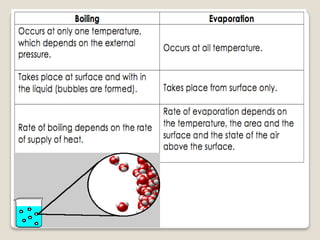
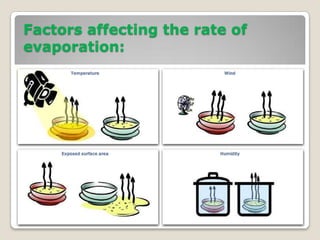
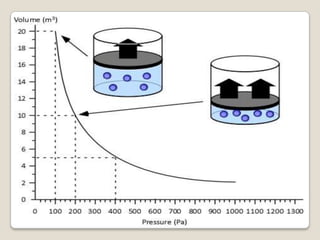
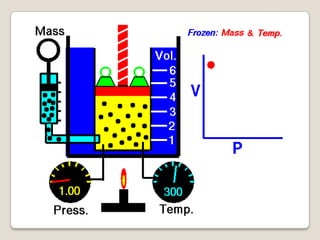
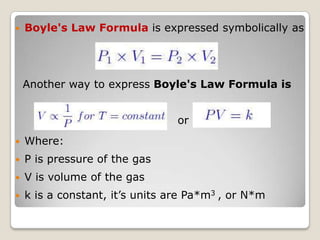
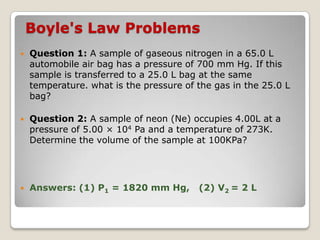
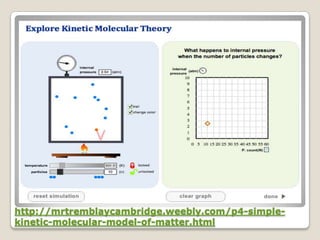
This document discusses the five states of matter: solid, liquid, gas, plasma, and Bose-Einstein condensate. It provides details on the properties and characteristics of each state, including that solids have a definite shape and volume, liquids have a definite volume but not shape, and gases are easily compressed and have no definite shape or volume. Additional topics covered include kinetic theory, fluids, phase changes, how heat and pressure affect boiling points and freezing points, and Boyle's law regarding the inverse relationship between gas pressure and volume.