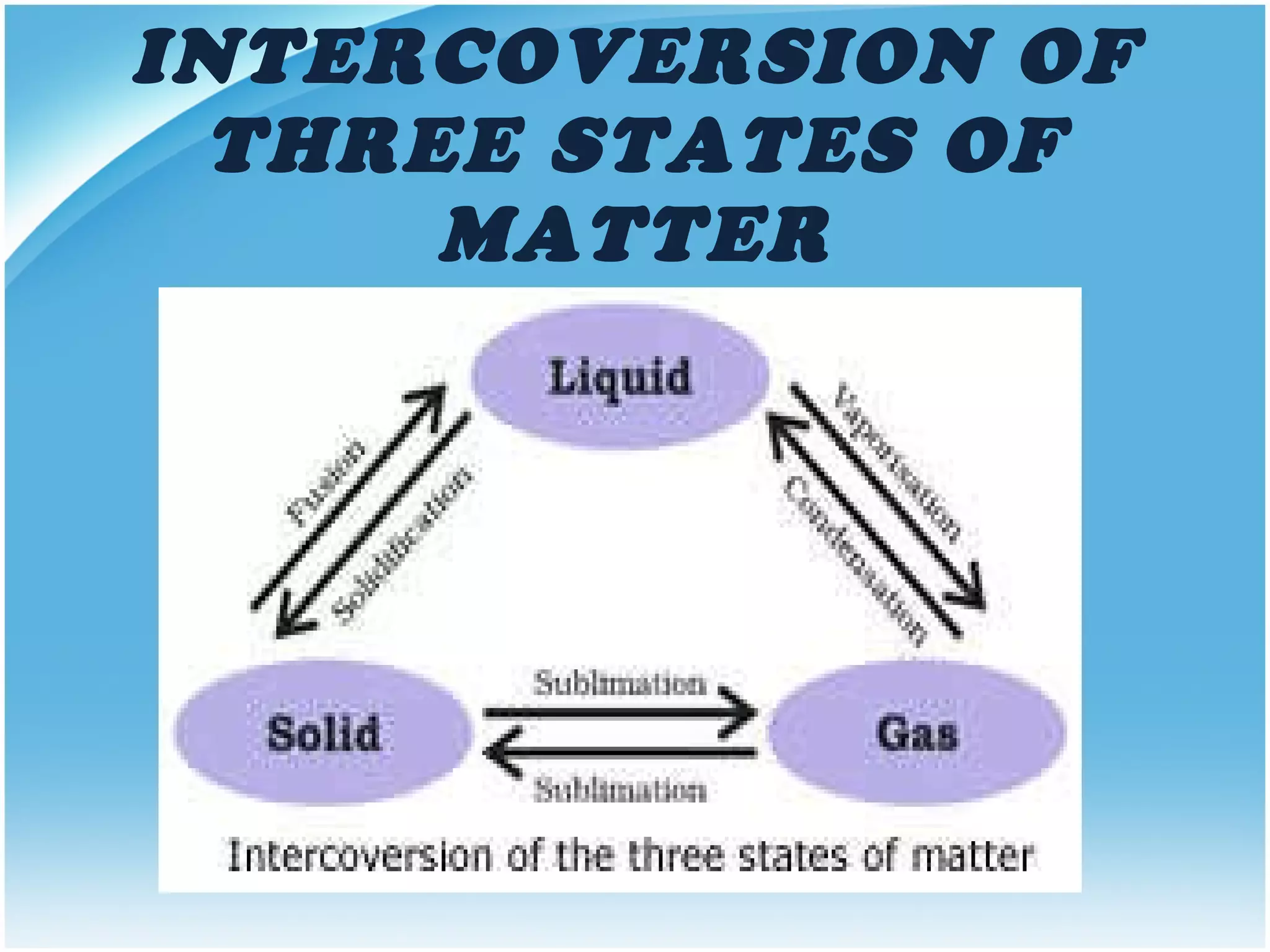
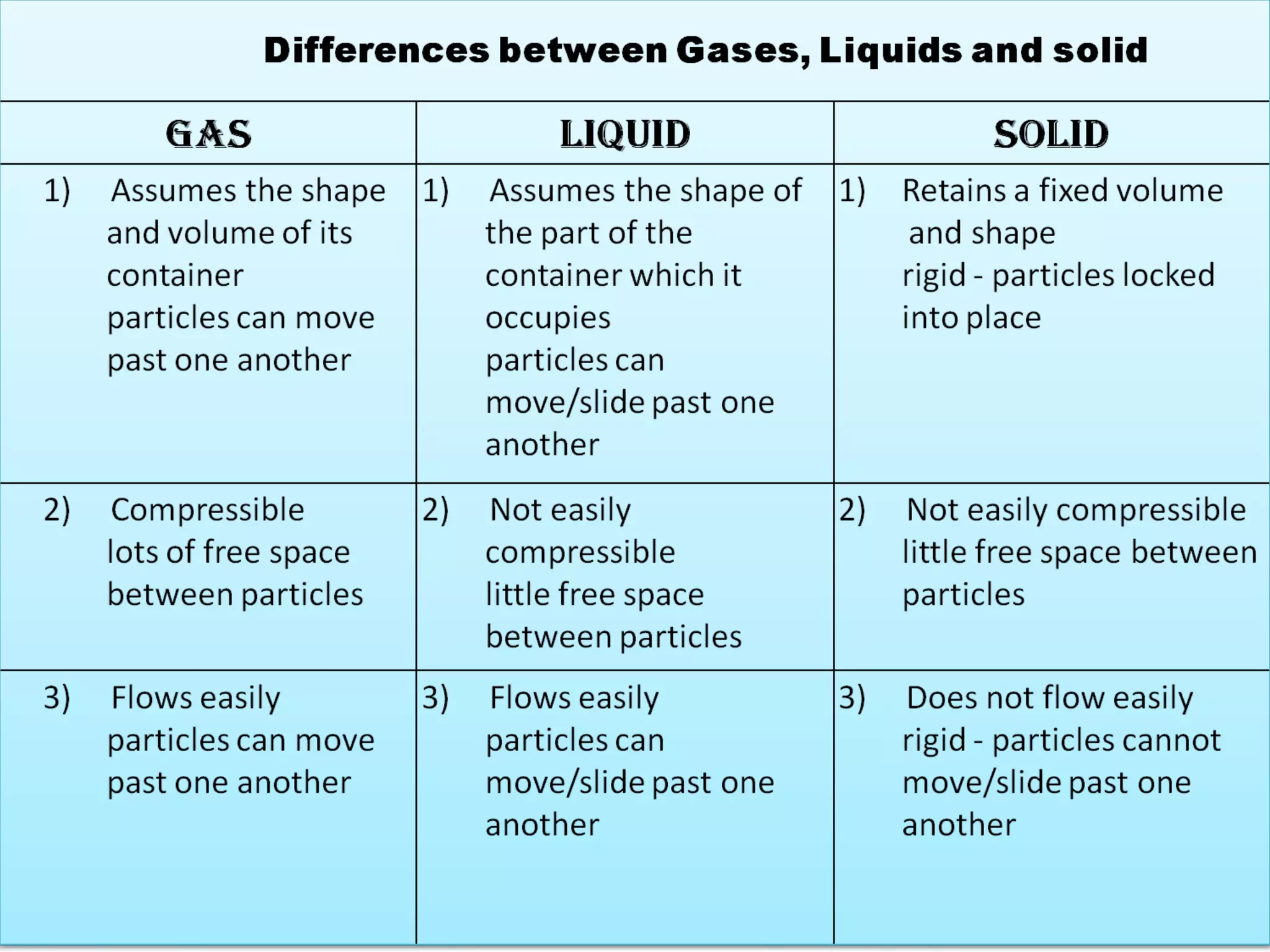
Matter can exist in several states including solid, liquid, gas, and plasma. The document defines these states and their key properties. Solids have a definite shape and volume, with particles that cannot move freely. Liquids flow and take the shape of containers but maintain a relatively constant density. Gases have the greatest distance between particles and are highly compressible with particles in random motion. Higher temperatures can change the state from solid to liquid to gas or ionize it into plasma.