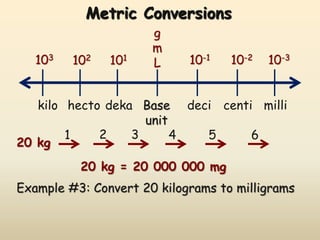
Physics is the study of the natural world and how physical objects behave. It began in ancient Greece when early scientists called "physikoi" tried to understand the natural world using observations and experiments. Today, physics involves measuring various quantities accurately using standardized metric units like meters, kilograms, and seconds. Measurements in physics consist of a number and a unit, and the International System of Units (SI) precisely defines the fundamental base units and derived units used in physics.