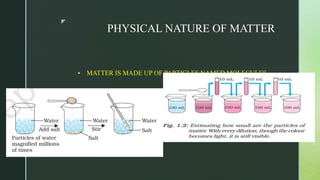
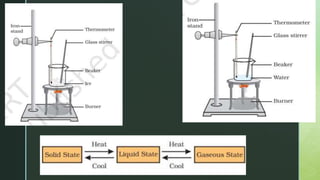
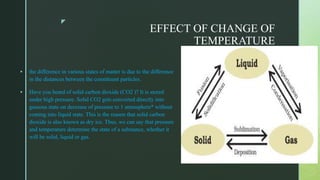
The document discusses the physical nature of matter. It states that everything in the universe is made up of matter which is composed of particles called molecules. Matter can exist in three states - solid, liquid and gas - depending on the strength of attraction between the particles and how freely they can move. A change in state, such as from solid to liquid to gas, requires an addition or removal of heat. This absorption or release of heat is known as latent heat and allows transformations like melting, boiling and evaporation to occur without changing temperature. Evaporation is defined as the process where liquid particles near the surface gain energy and change to gas below the boiling point of the liquid.