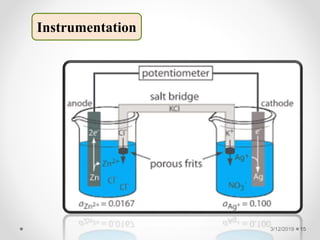
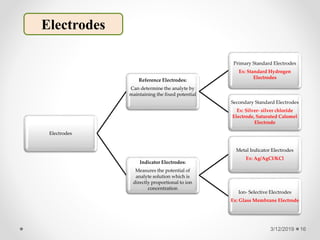
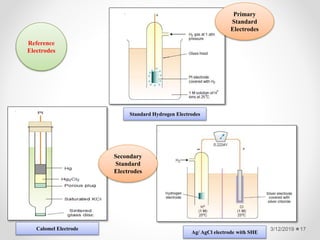
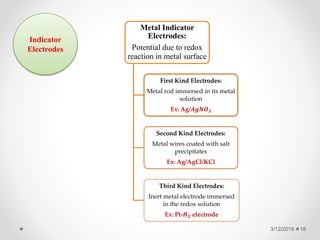
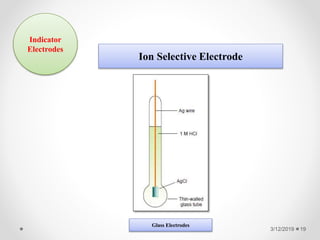
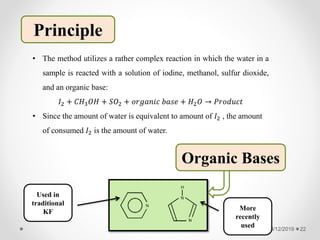
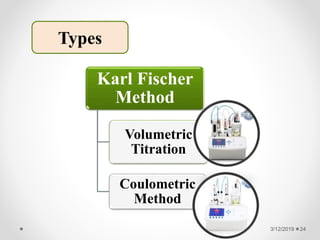
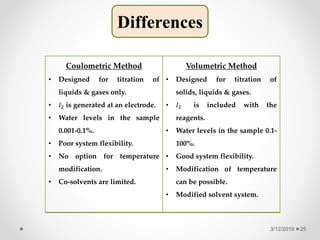
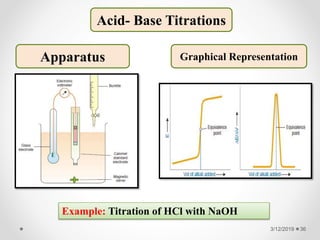
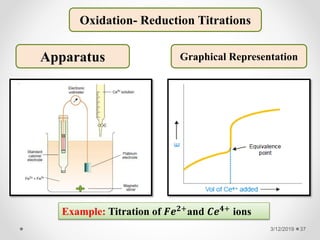
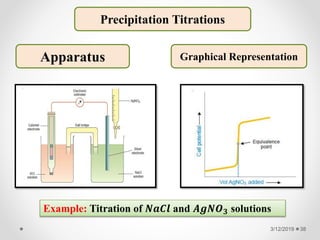
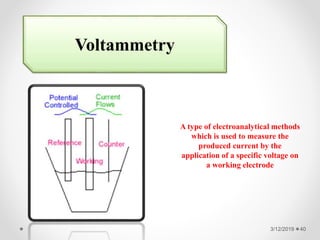
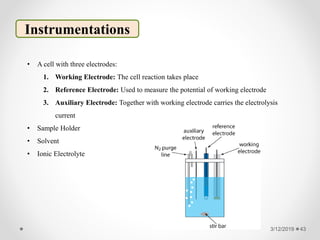
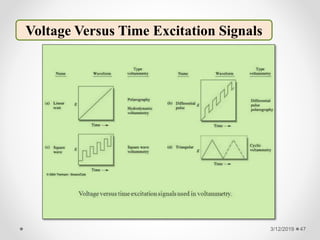
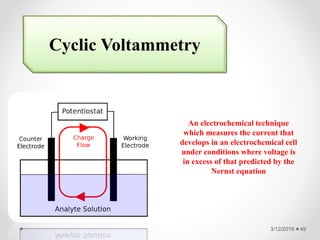
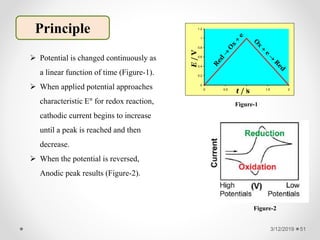
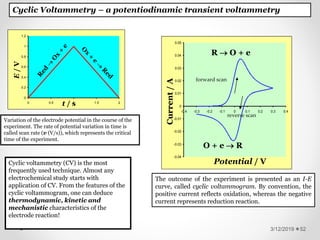
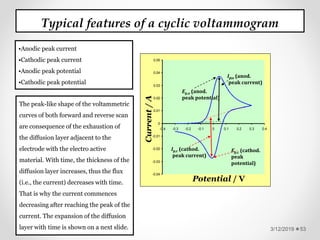
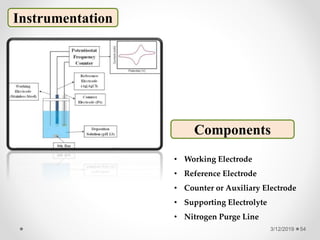
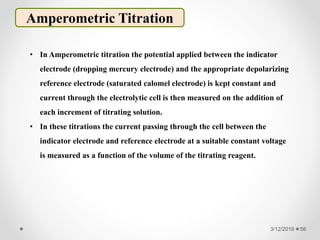
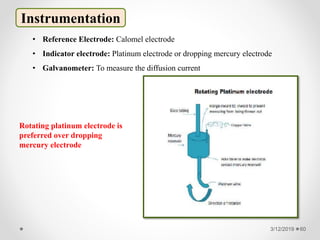
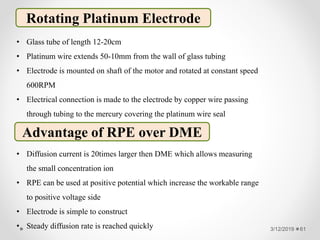
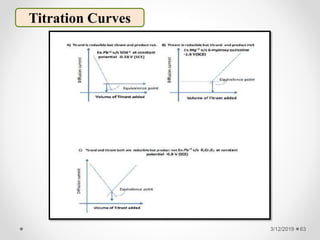
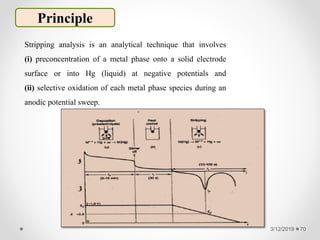
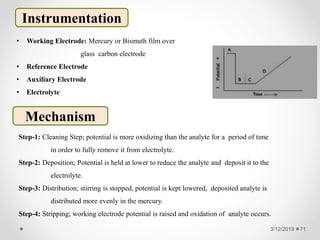
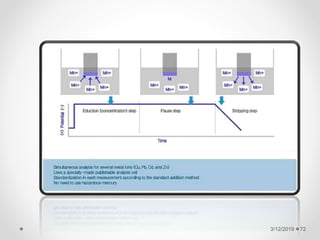
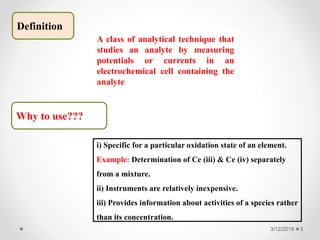
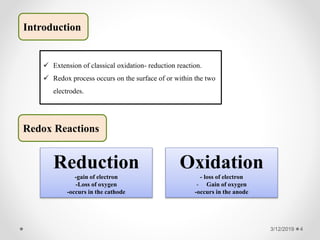
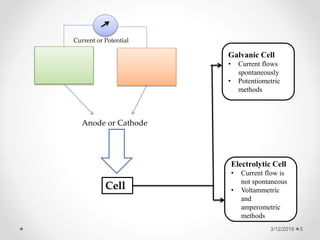
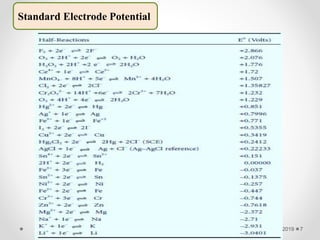
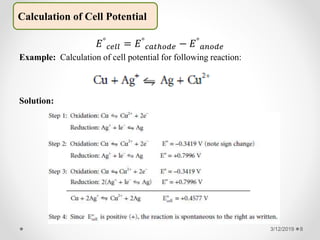
The document provides information about electroanalytical methods of analysis. It defines electroanalytical methods as techniques that study analytes by measuring potentials or currents in an electrochemical cell containing the analyte. It discusses various types of electroanalytical techniques including potentiometry, voltammetry, and Karl Fischer titration. It provides details on the principles, instrumentation, applications, and advantages of these analytical methods.

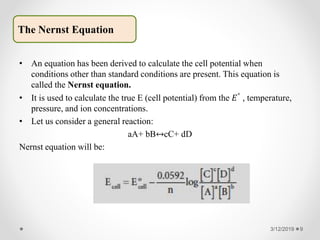
![What is the E for the 𝐹𝑒3+/𝐹𝑒2+ half cell if [𝐹𝑒3+] = 10−4M and [𝐹𝑒2+] =
10−1M at 25℃?
Solution:
3/12/2019 10
Calculation of Cell Potential By Nernst Equation](https://image.slidesharecdn.com/shanta1214016-190312064712/85/Electroanalytical-Methods-of-analysis-10-320.jpg)