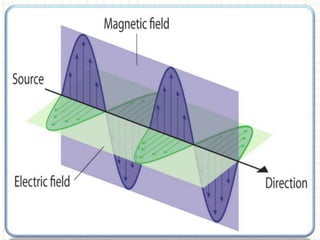
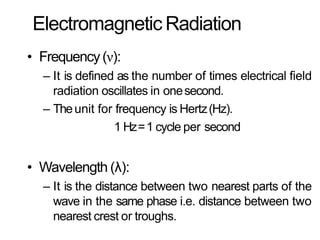
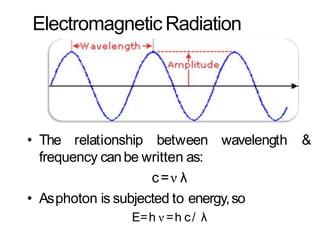
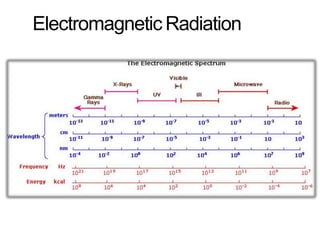
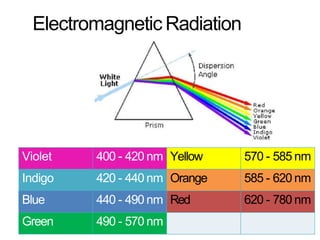
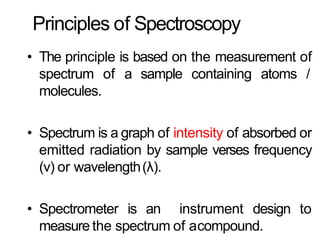
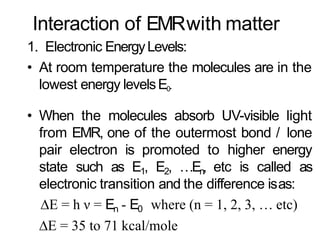
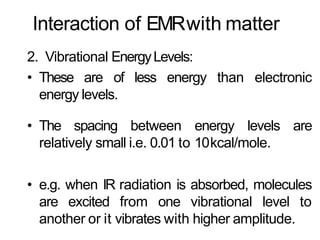
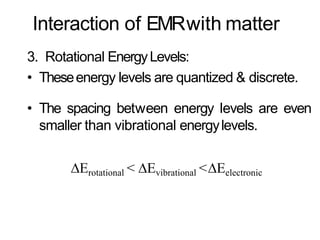
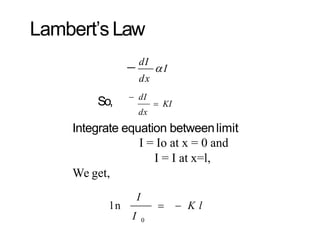
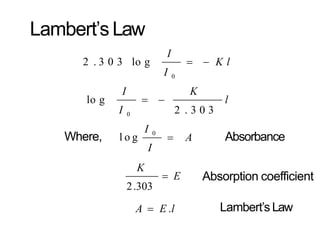
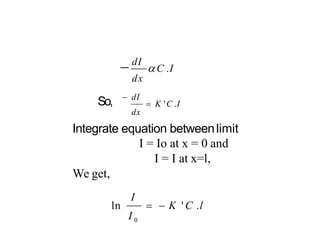
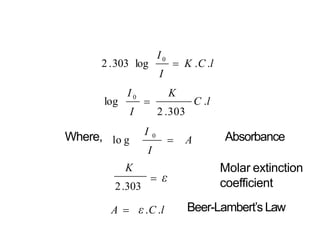
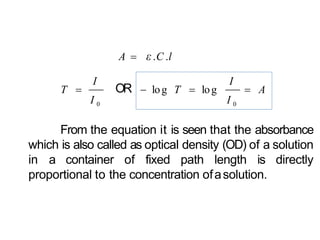
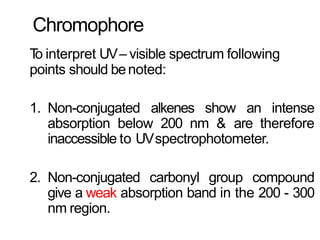
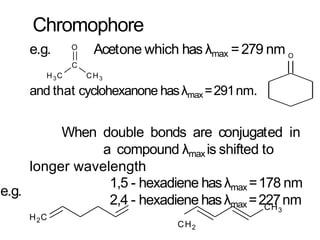
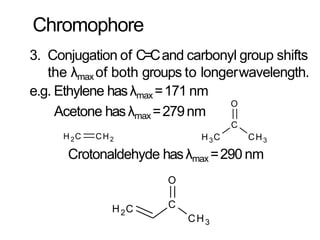
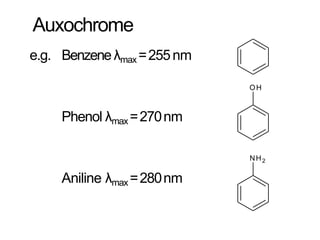
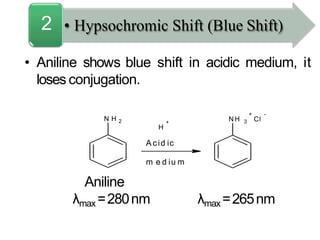
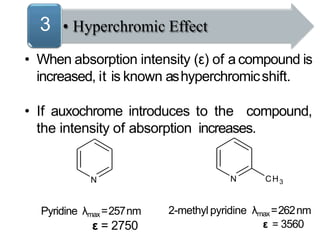
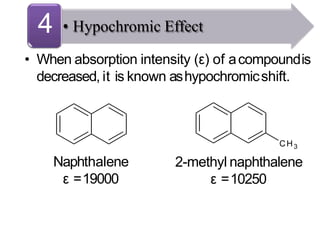
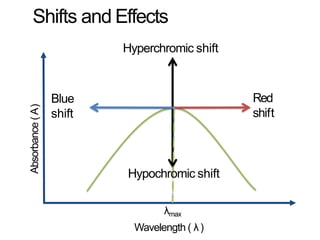
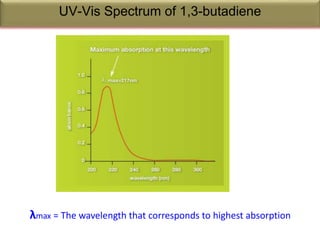
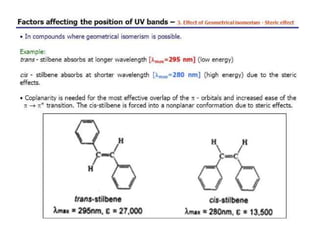
Spectroscopy is the branch of science that deals with the study of interaction of electromagnetic radiation with matter. It uses electromagnetic radiation in the ultraviolet-visible region. When this radiation interacts with molecules, electronic transitions between different energy levels can occur. The wavelength and intensity of absorbed light depends on characteristics of the molecule such as its structure and functional groups. Spectroscopy can be used to identify unknown compounds, determine molecular structure, and calculate concentration through the Beer-Lambert law.