This document discusses symmetry elements and symmetry operations in group theory and chemistry. It defines the key symmetry elements - identity (E), proper rotation axes (Cn), mirror planes (σ), inversion centers (i), and improper rotation axes (Sn). It provides examples of how these elements apply to common molecular structures like water, methane, benzene, and transition metal complexes. The document also explains the different types of mirror planes and how the symmetry operations are generated from each element.


![Symmetry elements and symmetry operations :-
Symmetry Elements Symmetry Operations
1. Identity [E] Doing nothing
2. Proper Rotation axis or
Axis of Symmetry [Cn]
Rotation about the axis through
some
angle
3. Mirror Plane or
Plane of Symmetry []
Reflection about the plane
4. Inversion Centre or
Centre of Symmetry [ i ]
Inversion
{ inversion is a reflection about a
point}
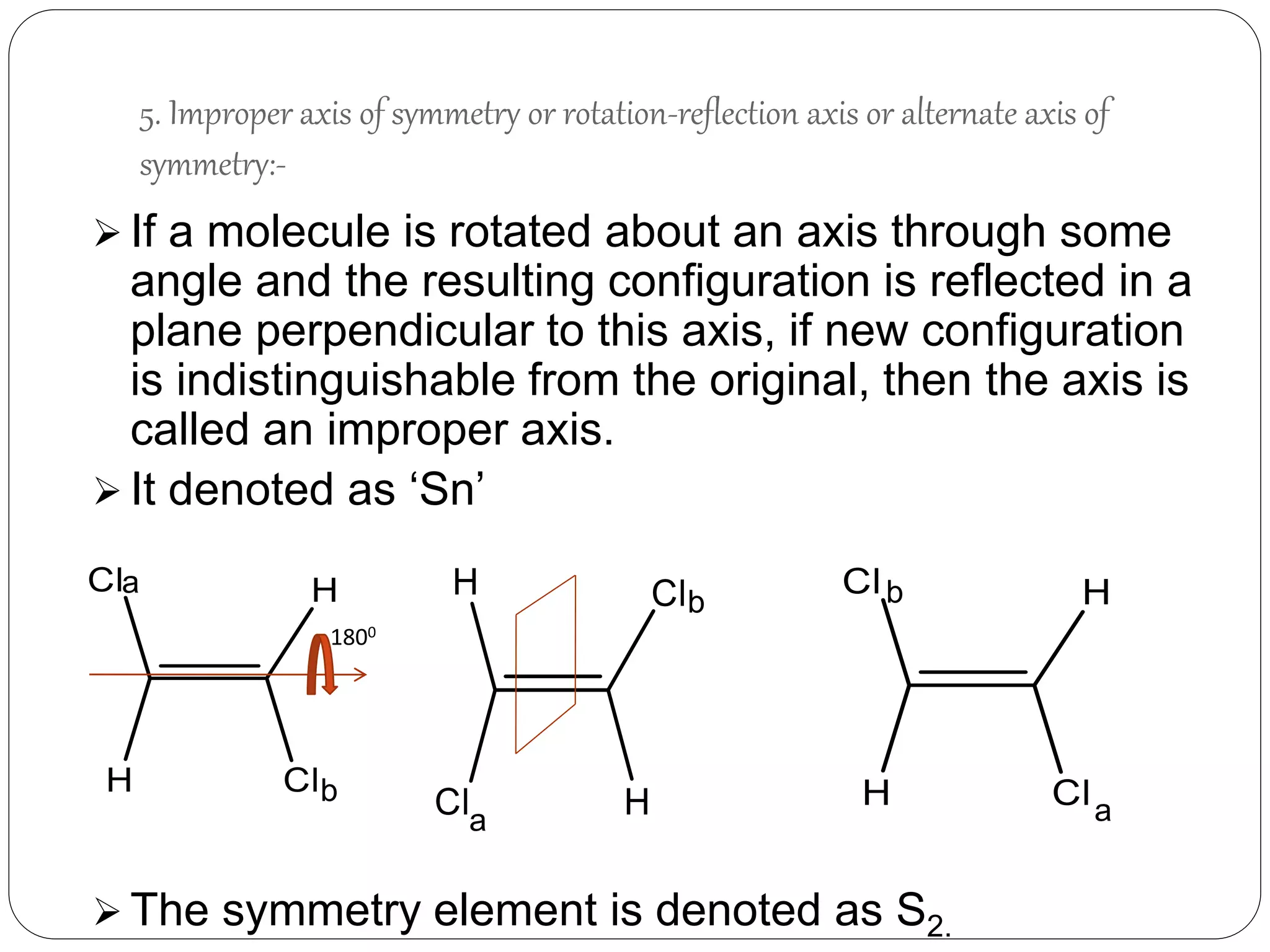
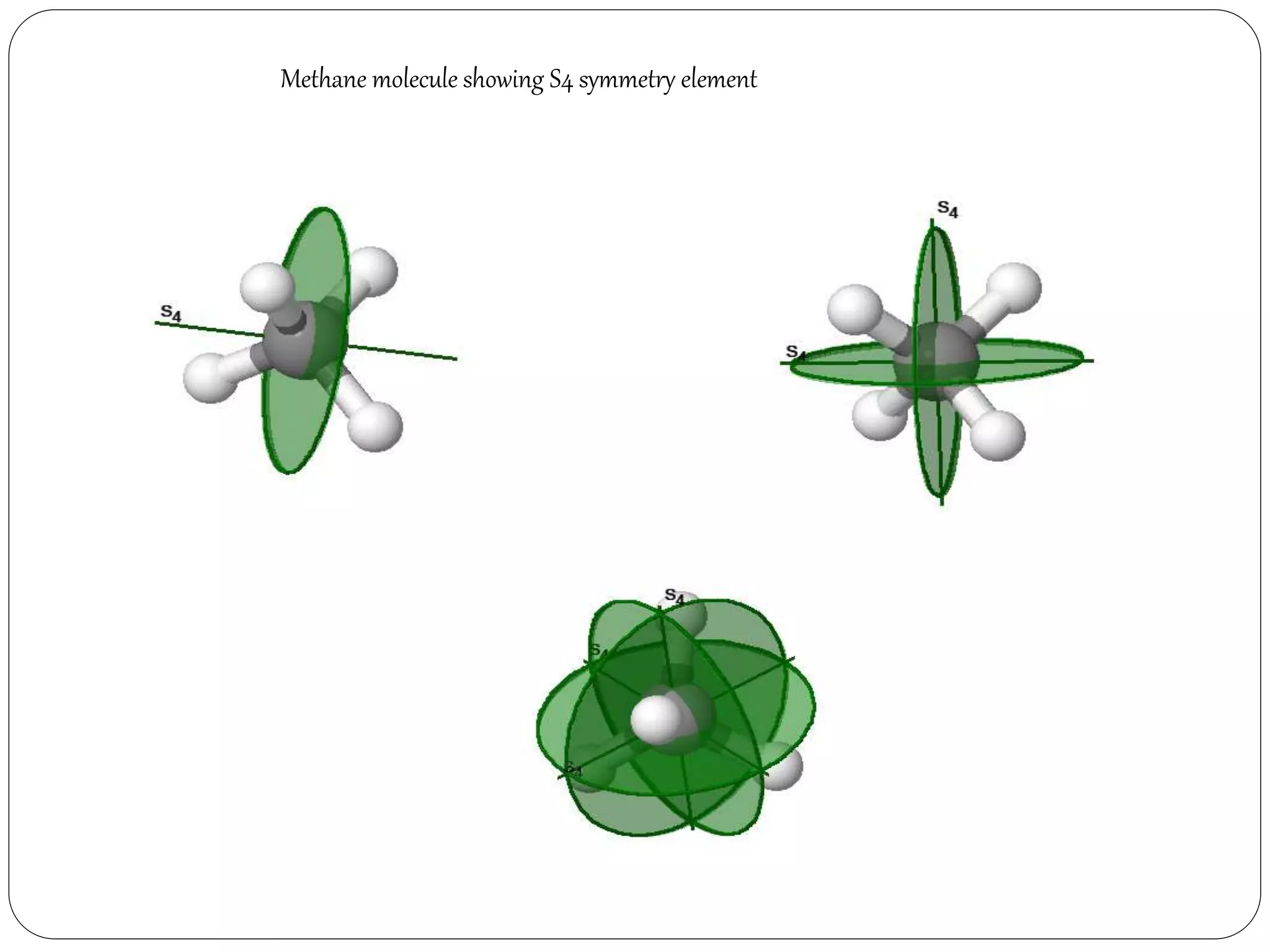
5. Improper Rotation axis or
Rotation- Reflection axis [Sn]
Rotation about an axis through
some
angle followed by a reflection in
a plane](https://image.slidesharecdn.com/presentation1-170330052820/75/SYMMETRY-ELEMENTS-AND-SYMMETRY-OPERATIONS-8-2048.jpg)
![1. Identity [E] :-
This is an operation which brings molecule back
to its original orientation.
This operation does nothing. It is simplest of all
the symmetry elements.
It is the only element/operation possessed by all
molecules.
It is denoted by E.
for example:- CHBrFCl](https://image.slidesharecdn.com/presentation1-170330052820/75/SYMMETRY-ELEMENTS-AND-SYMMETRY-OPERATIONS-9-2048.jpg)
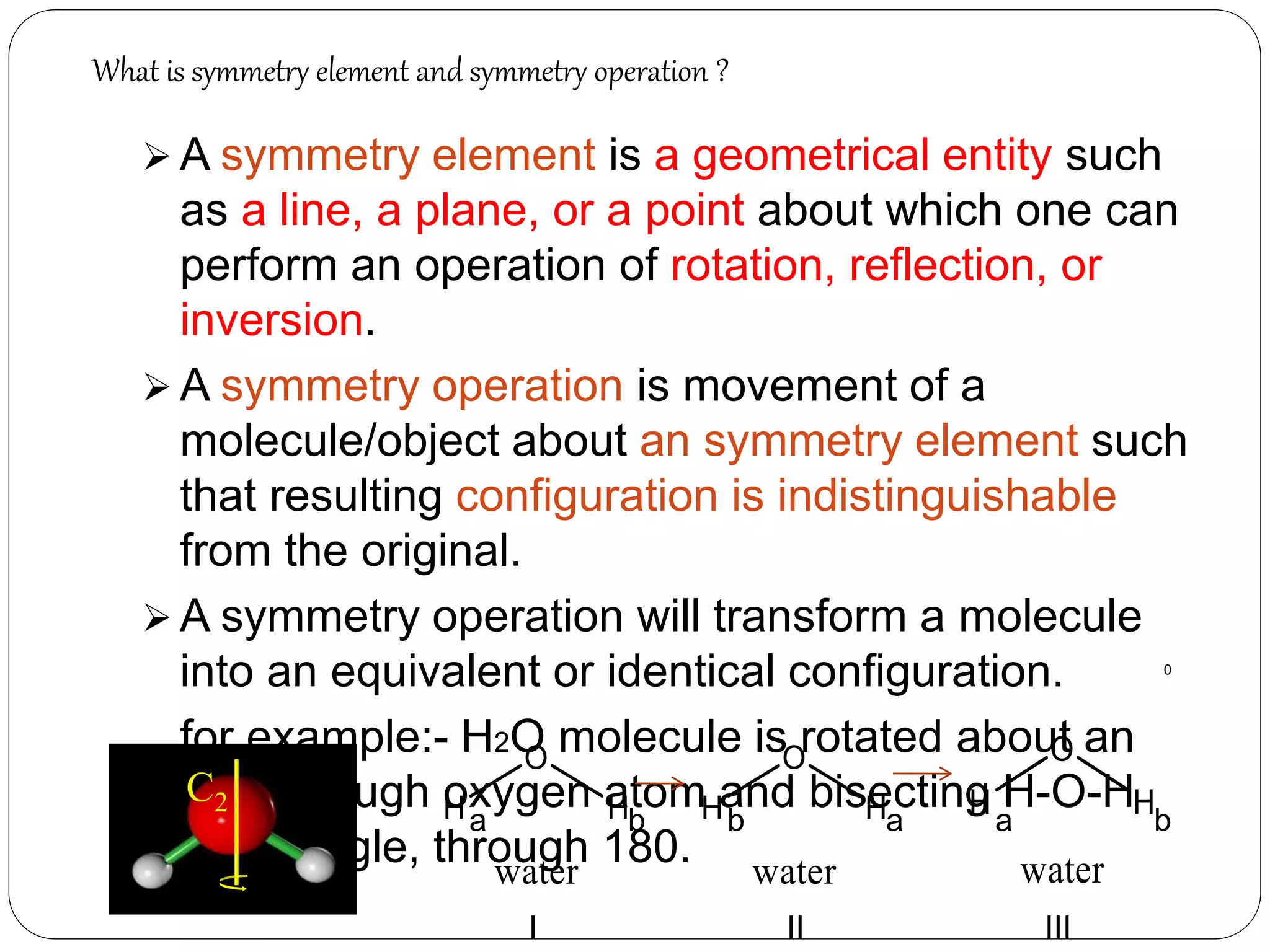
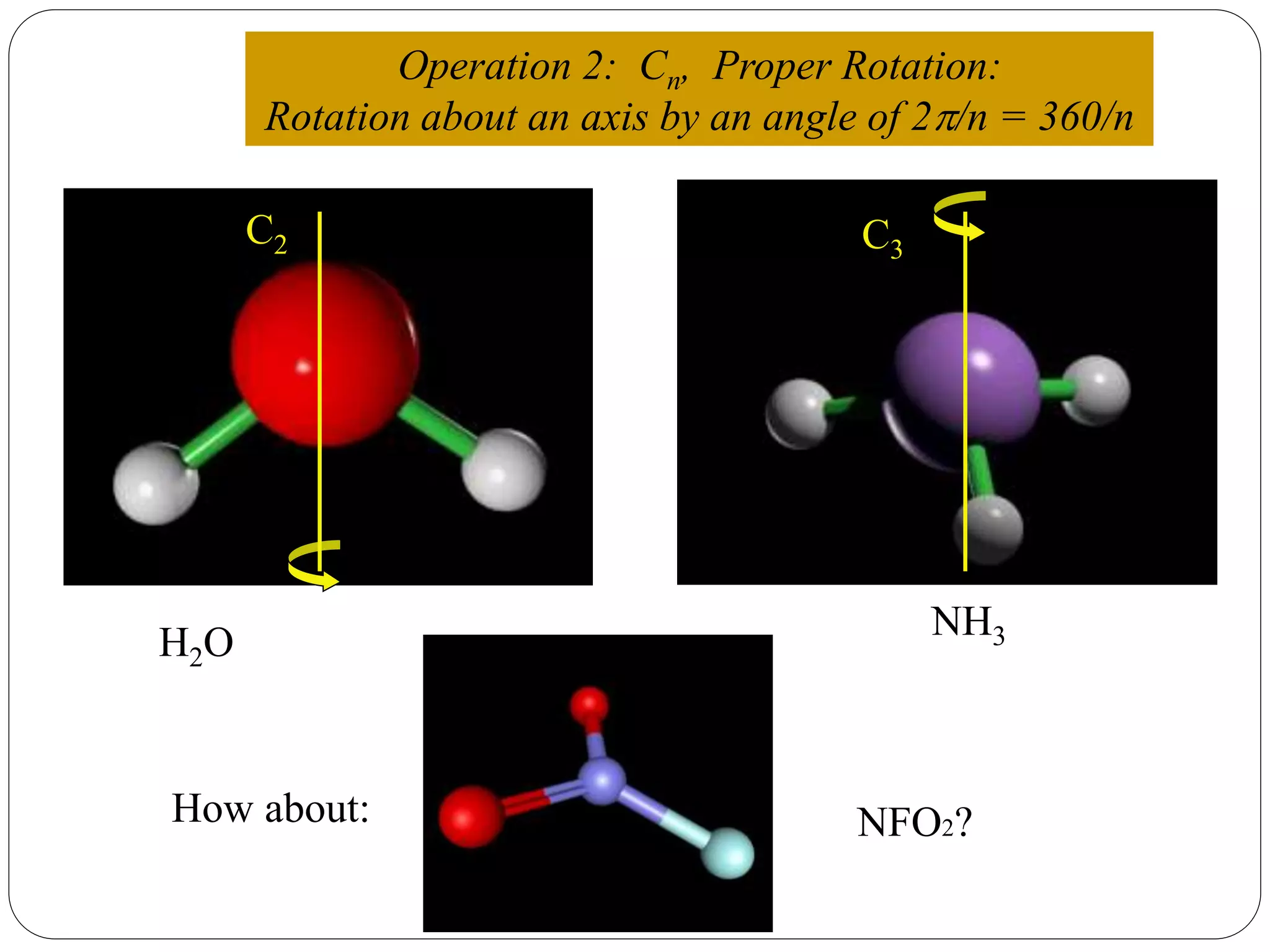
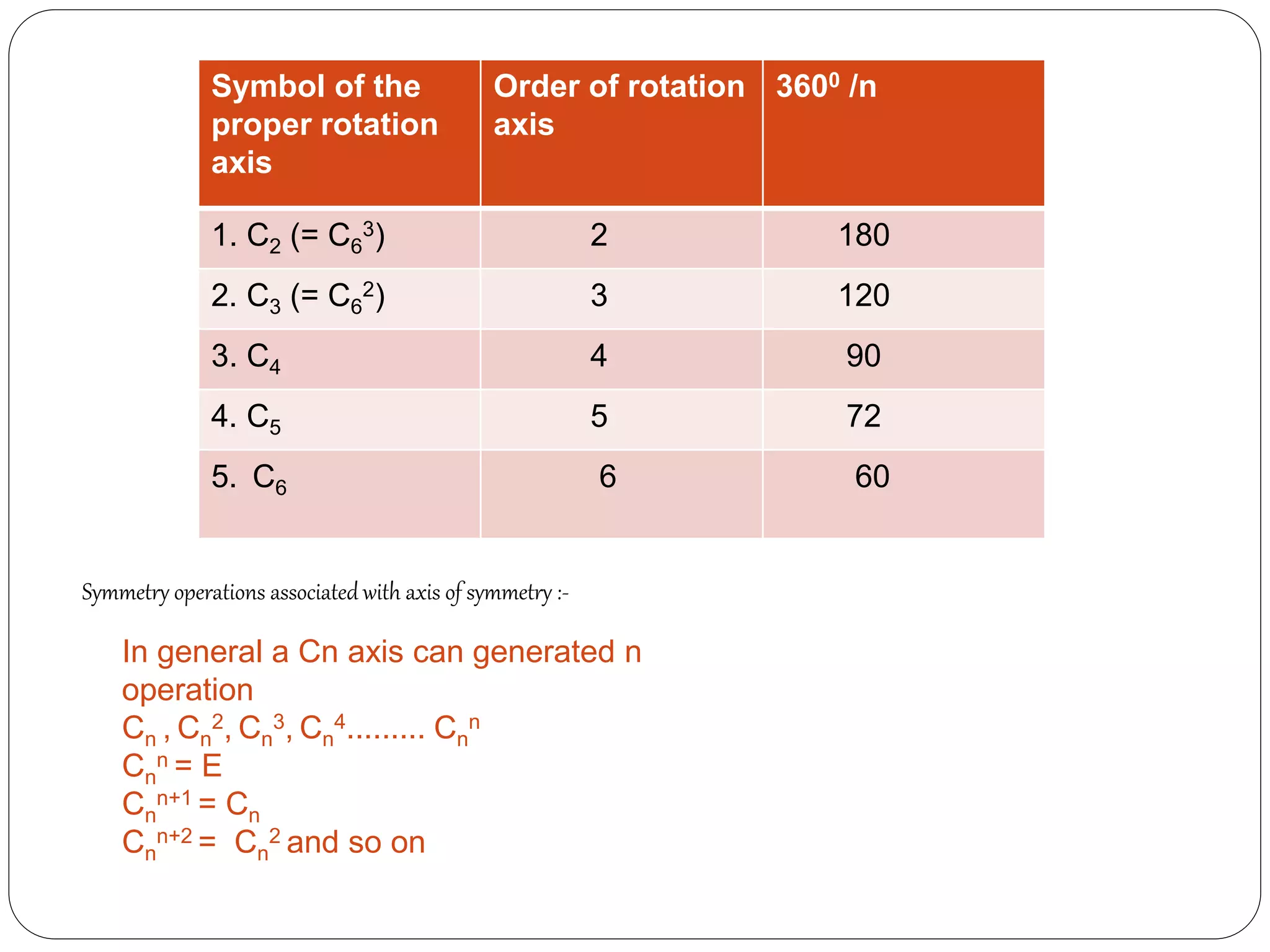
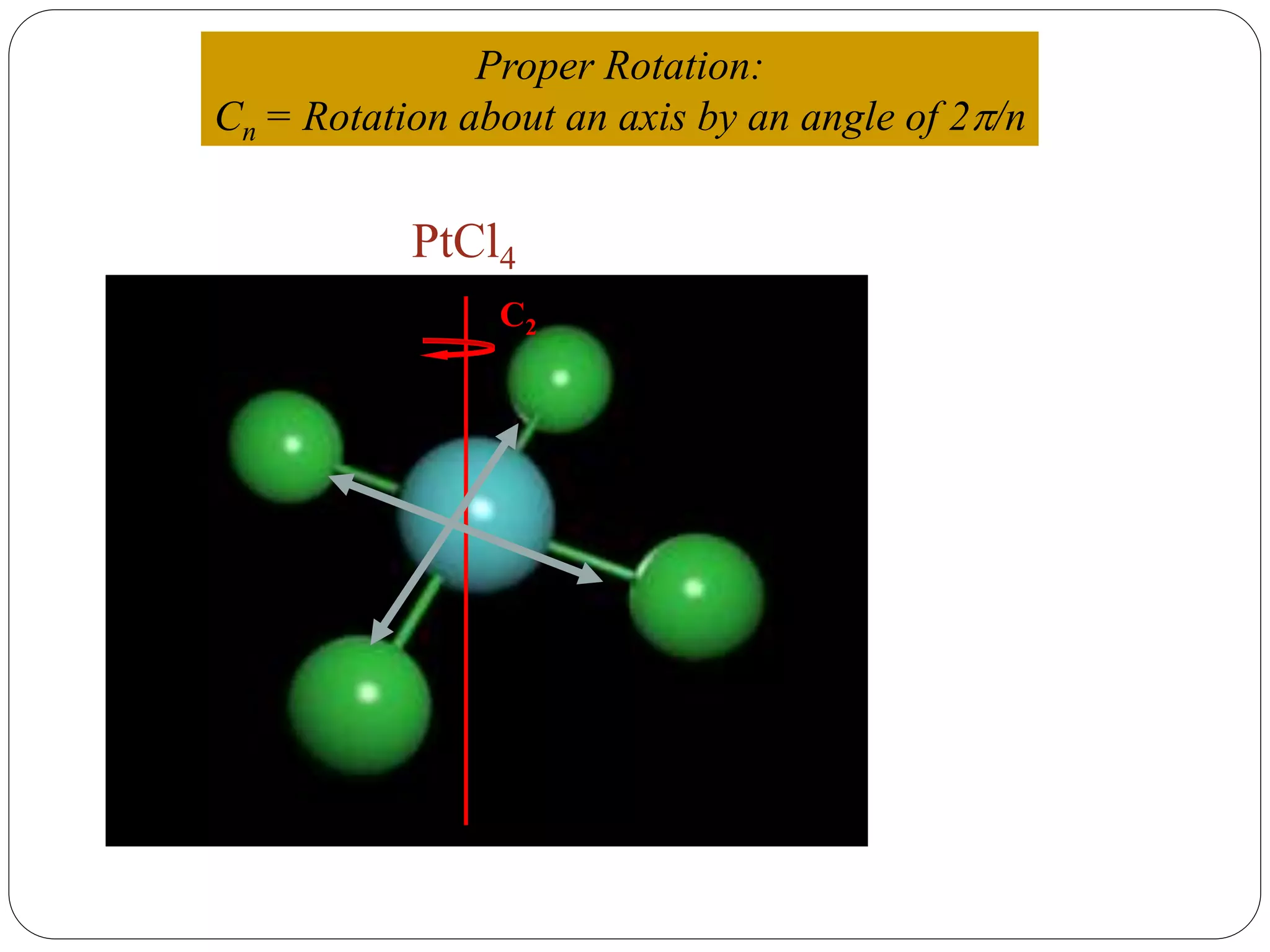
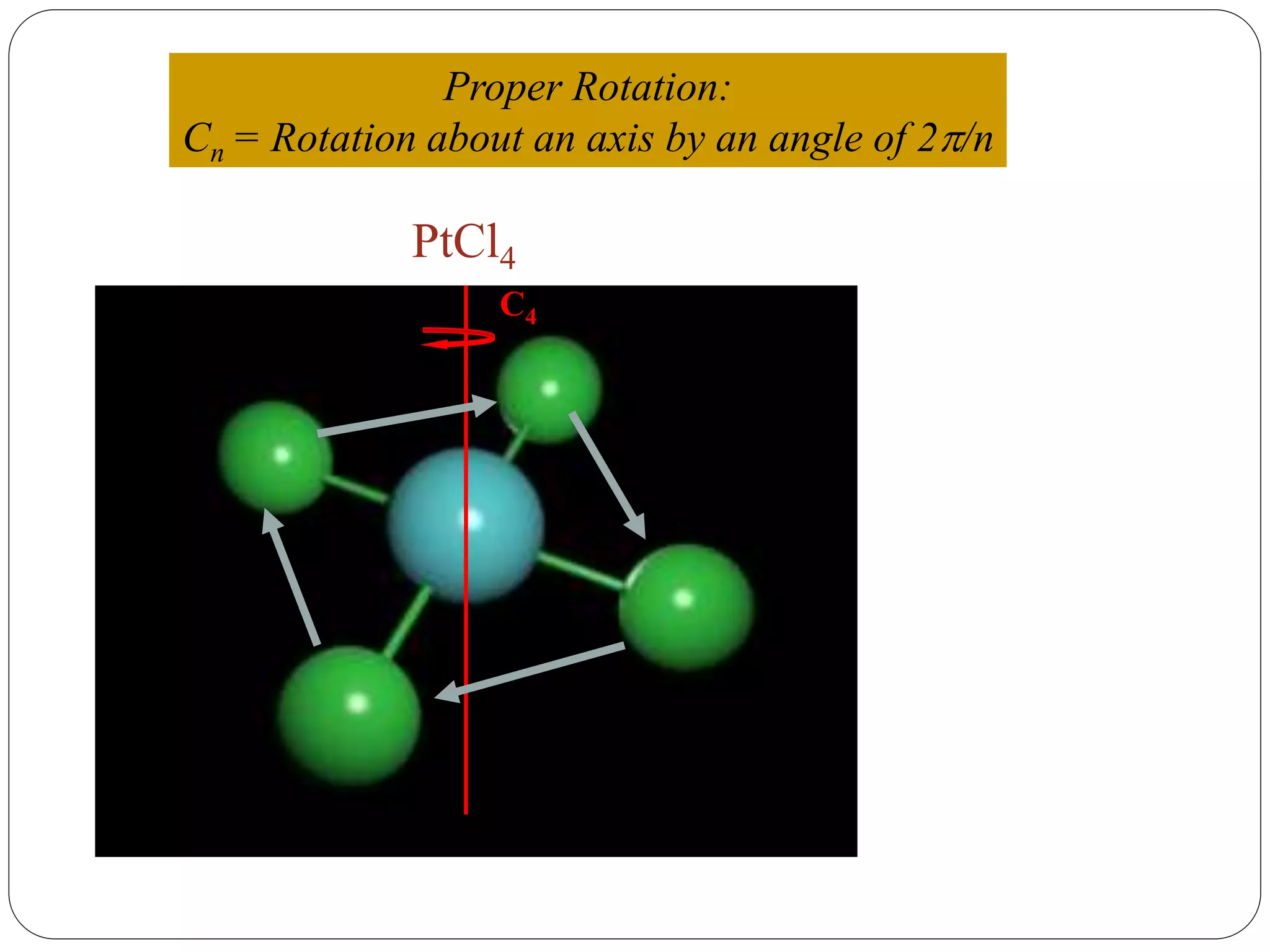
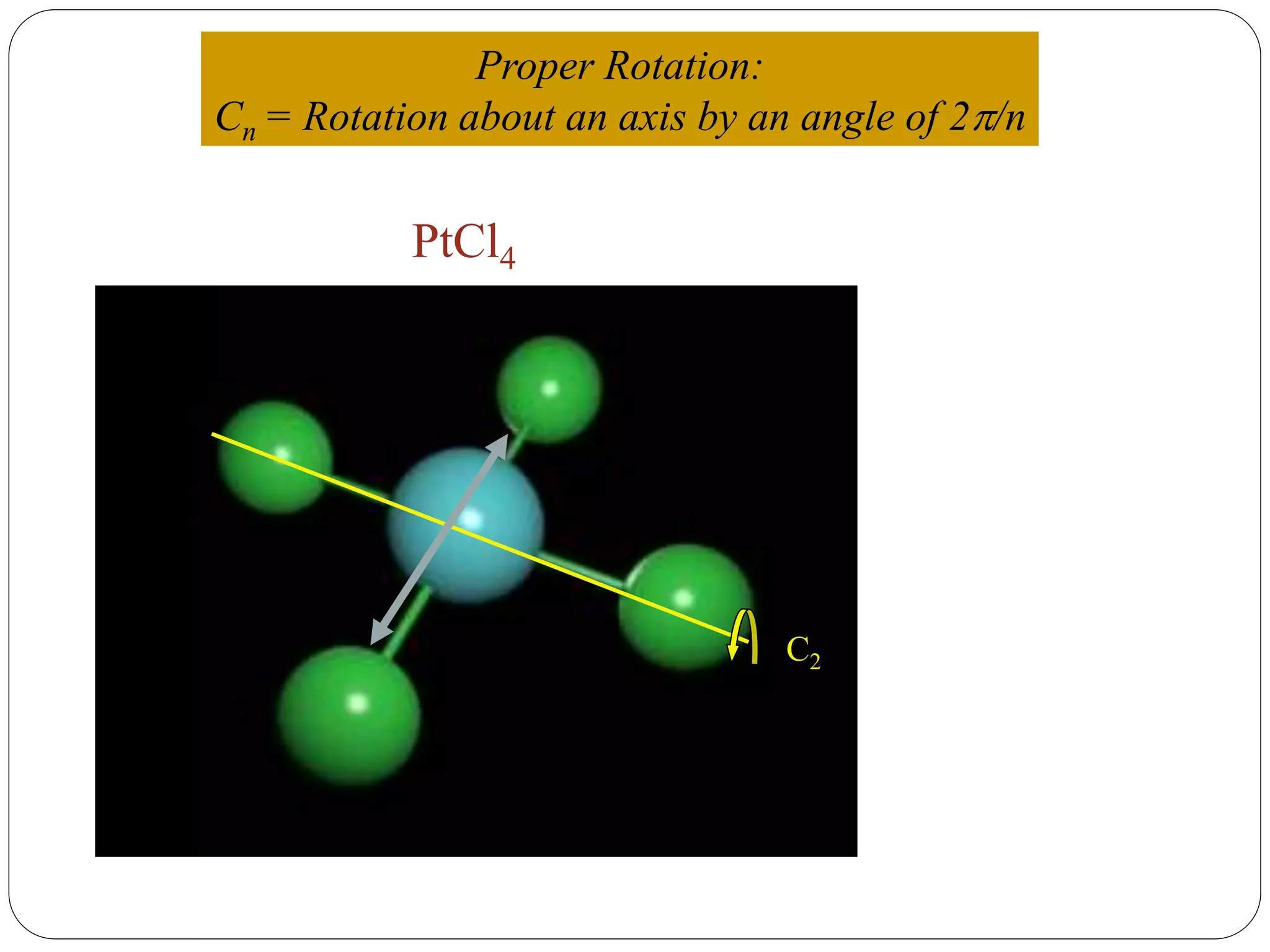
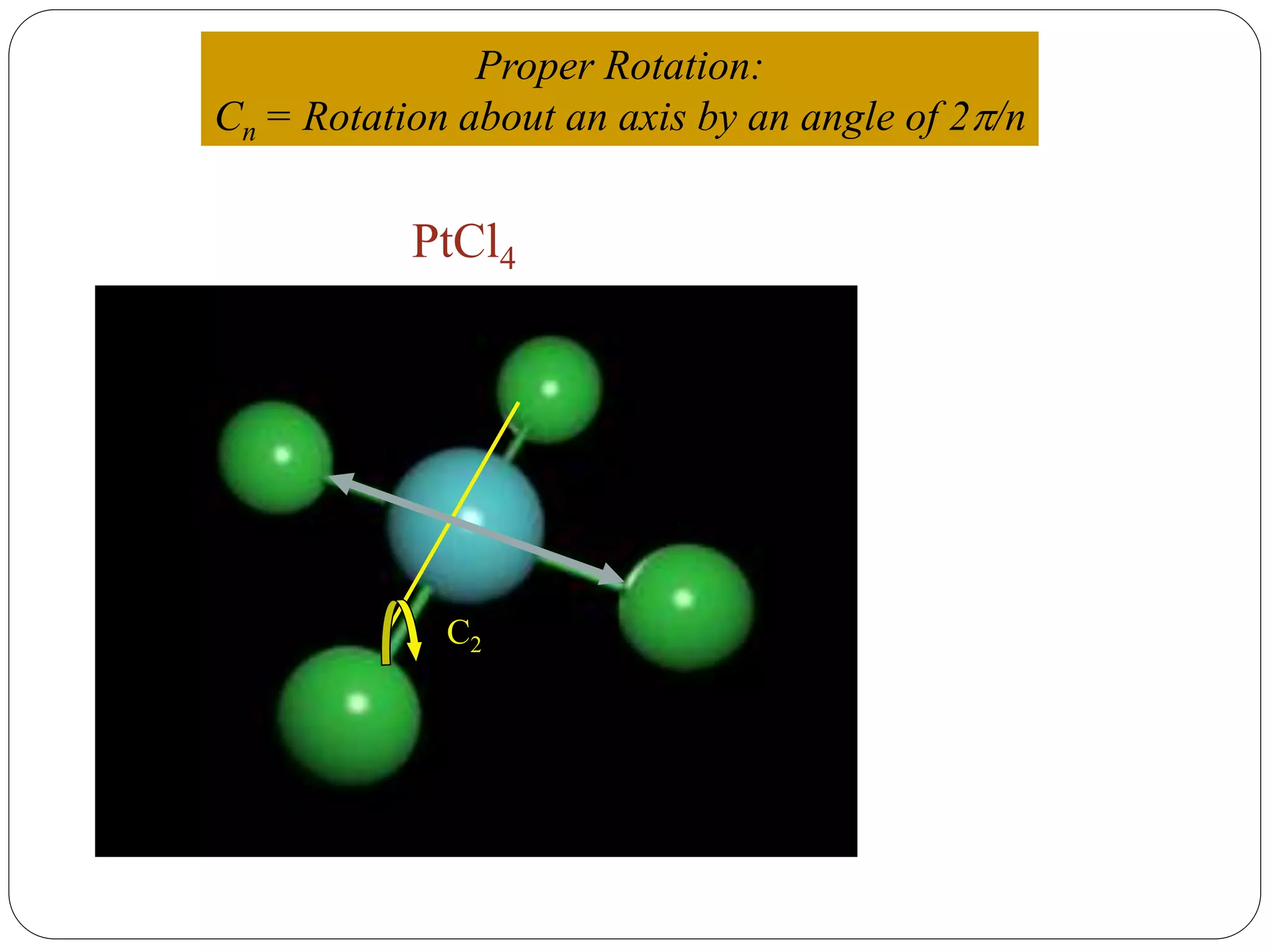
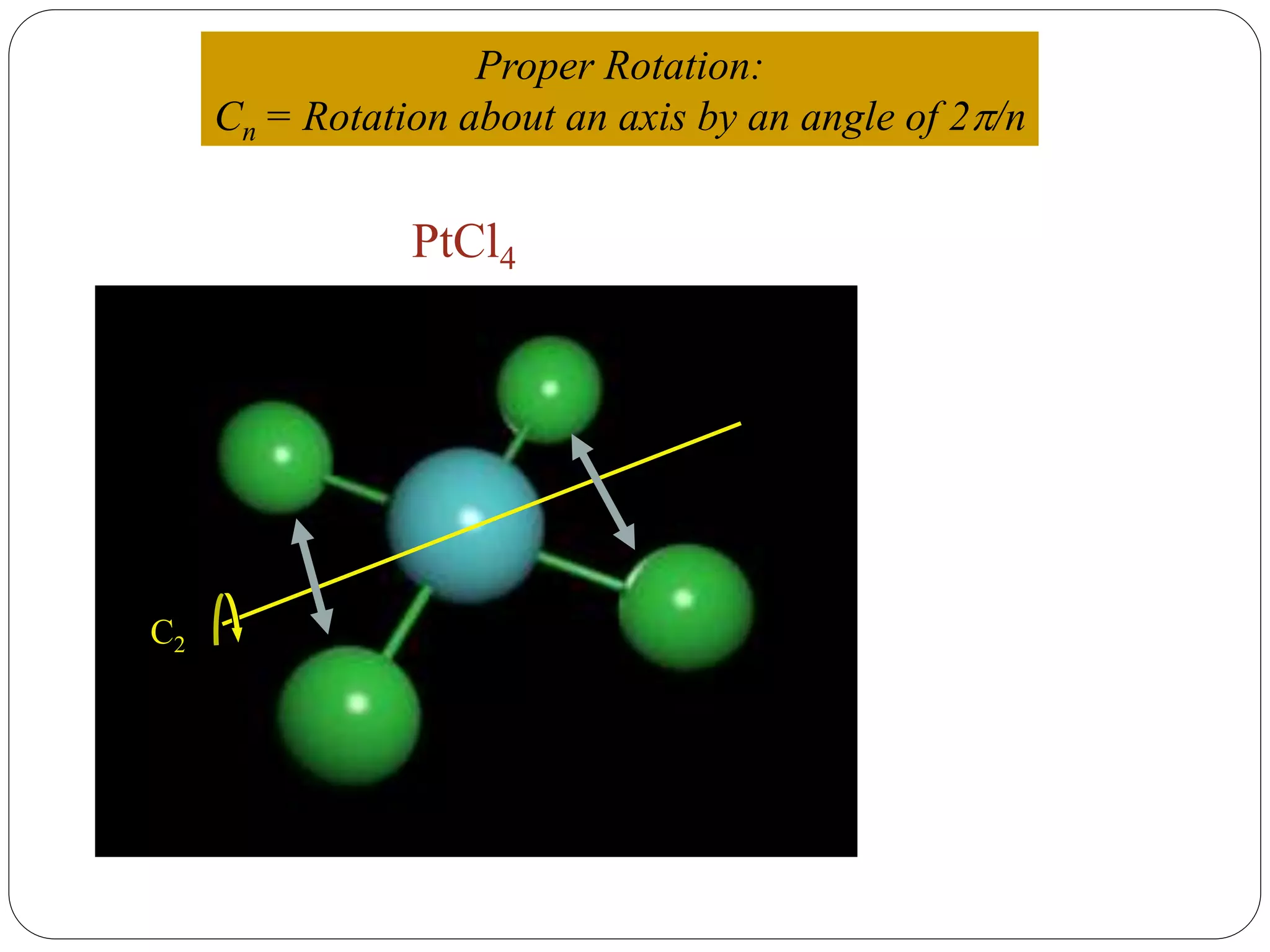
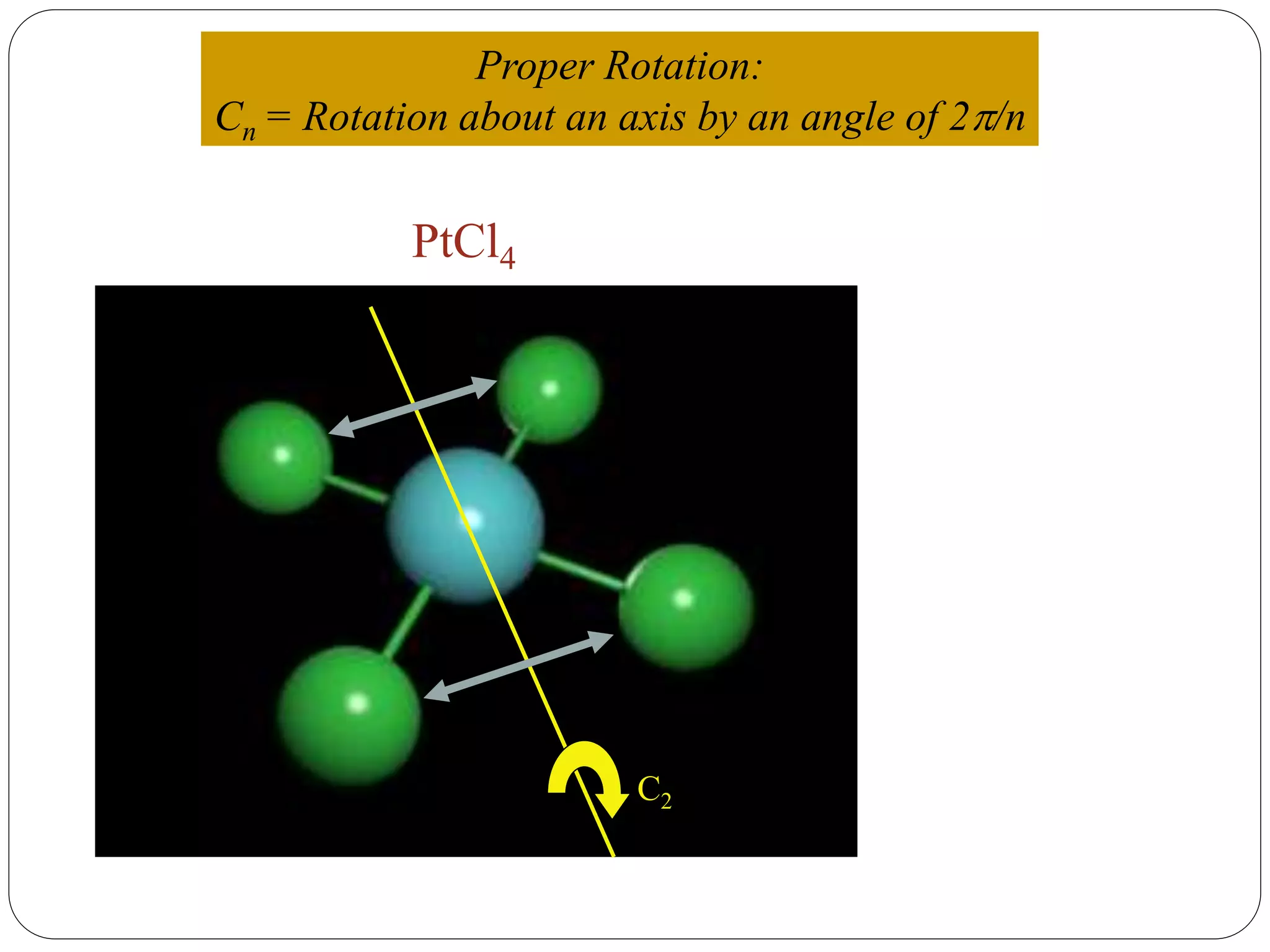
![2. Axis of symmetry [Cn] :-
It is called n-fold rotational axis.
If the rotation of a molecule about an axis through
some angle results in a configuration which is
indistinguishable from the original, then the
molecule is said to possess a proper rotation
axis.
It is denoted as Cn.
n is order of rotation axis.
Rotation about an axis by an angle of 360/n.
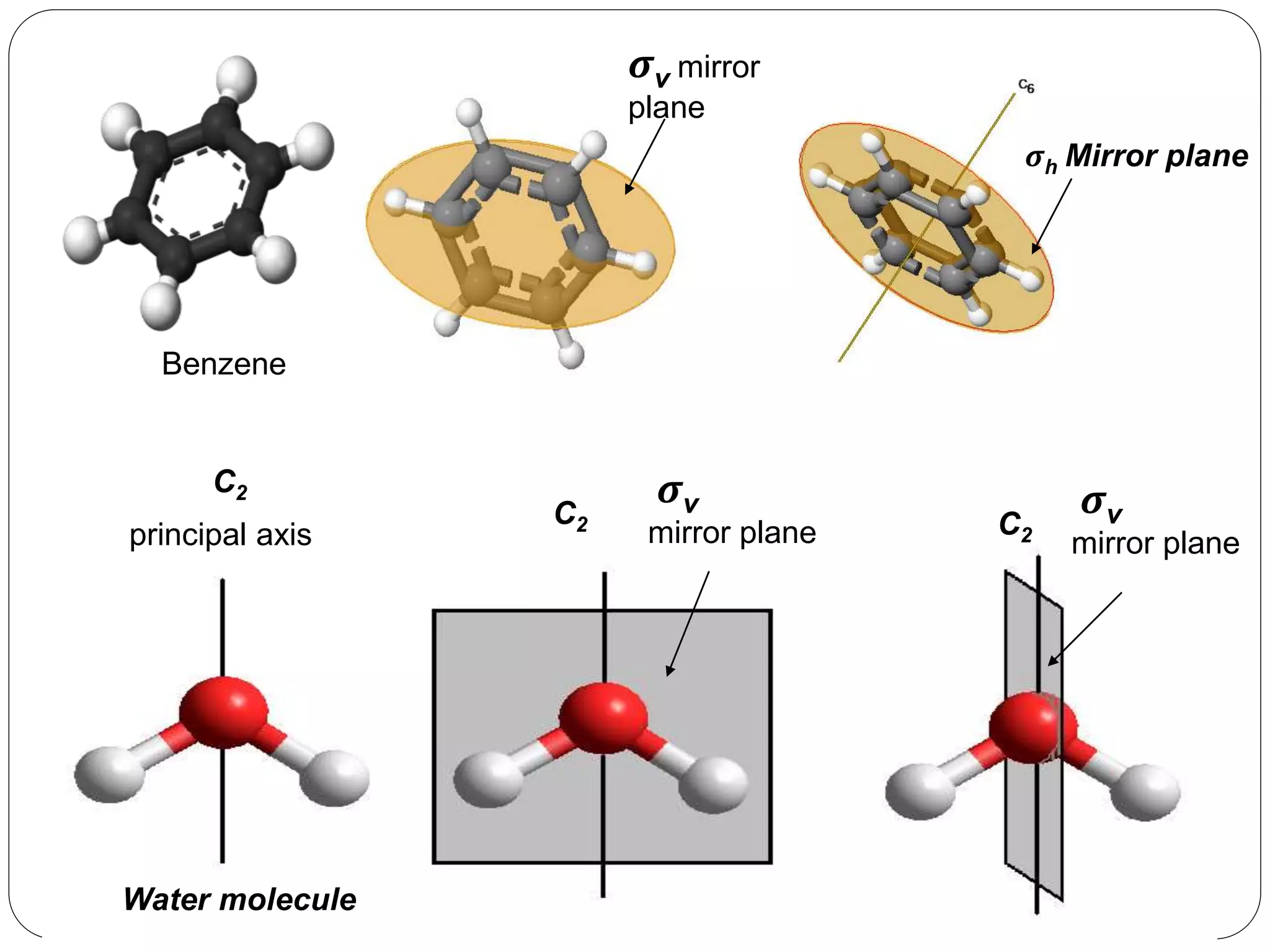
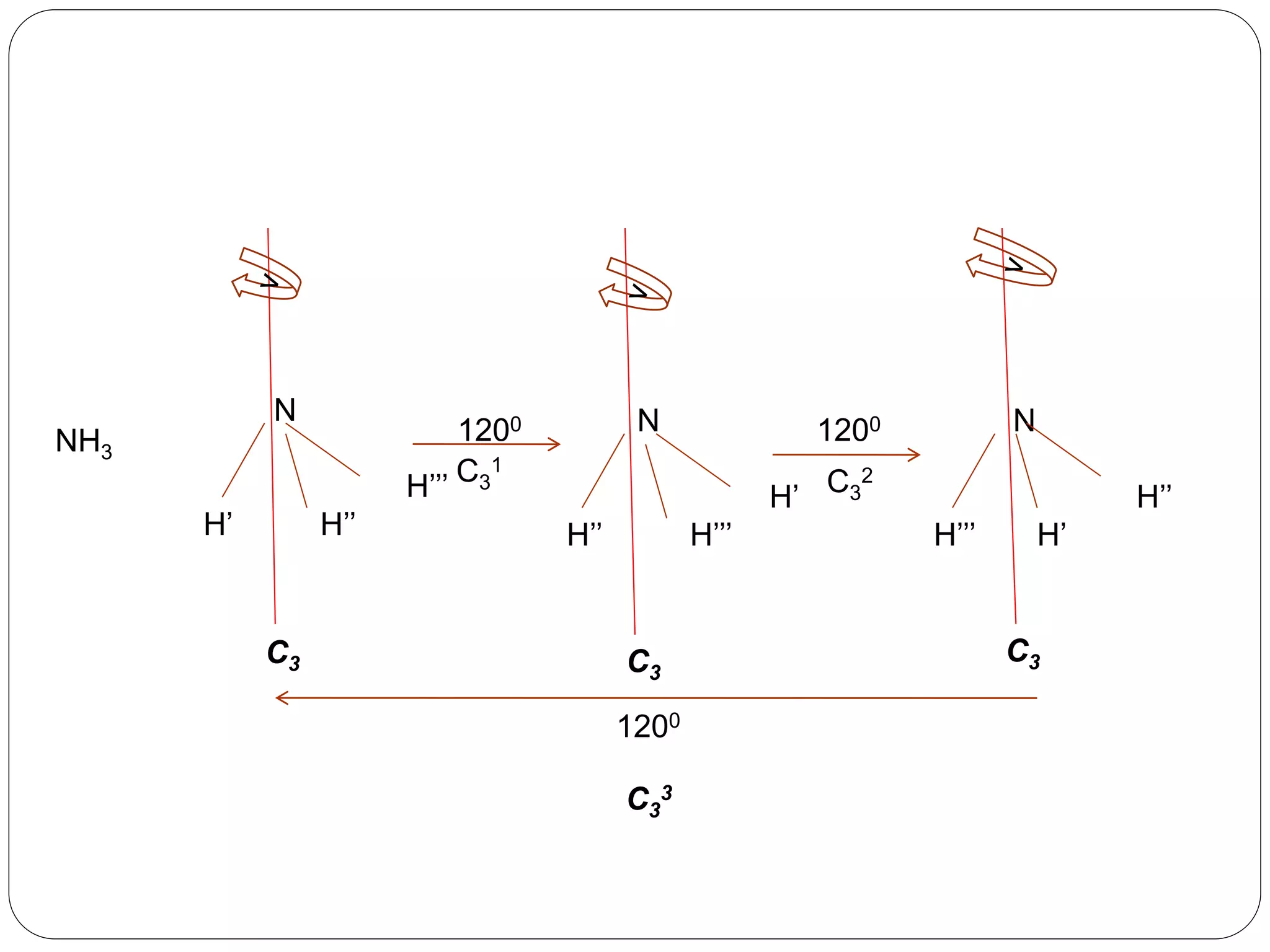
For example:- water molecule
a b
b a1800
Order of rotation axis = 2
Symmetry element = C2 axis
Operations = C2
1, C2
2 = E](https://image.slidesharecdn.com/presentation1-170330052820/75/SYMMETRY-ELEMENTS-AND-SYMMETRY-OPERATIONS-10-2048.jpg)
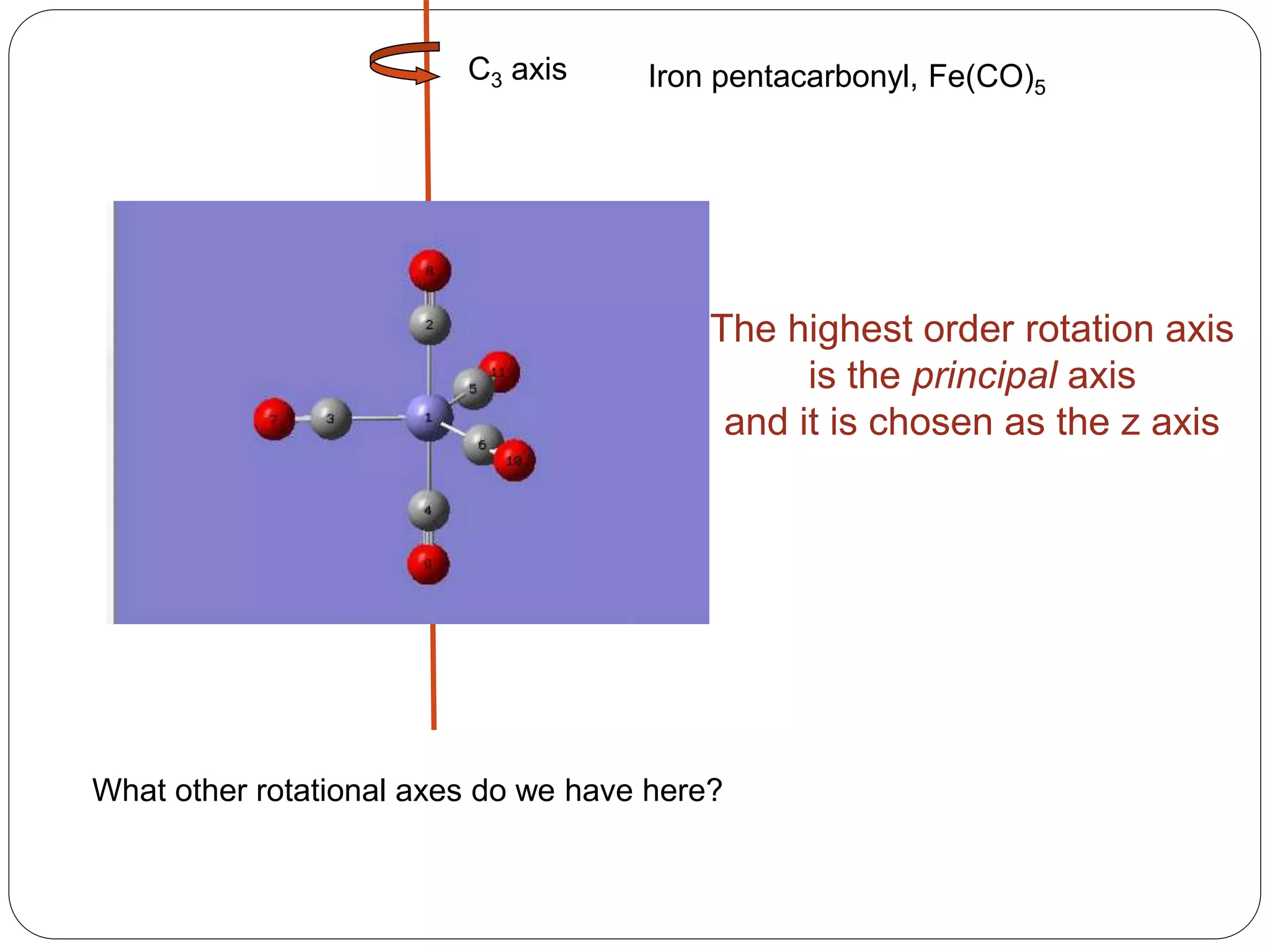
![BF3
F
B F
F
Ni
C3 C4 C6
F
B F
F
Niv
3 C2
4 C2 6 C2
Ni[CN]4 C6H6](https://image.slidesharecdn.com/presentation1-170330052820/75/SYMMETRY-ELEMENTS-AND-SYMMETRY-OPERATIONS-13-2048.jpg)
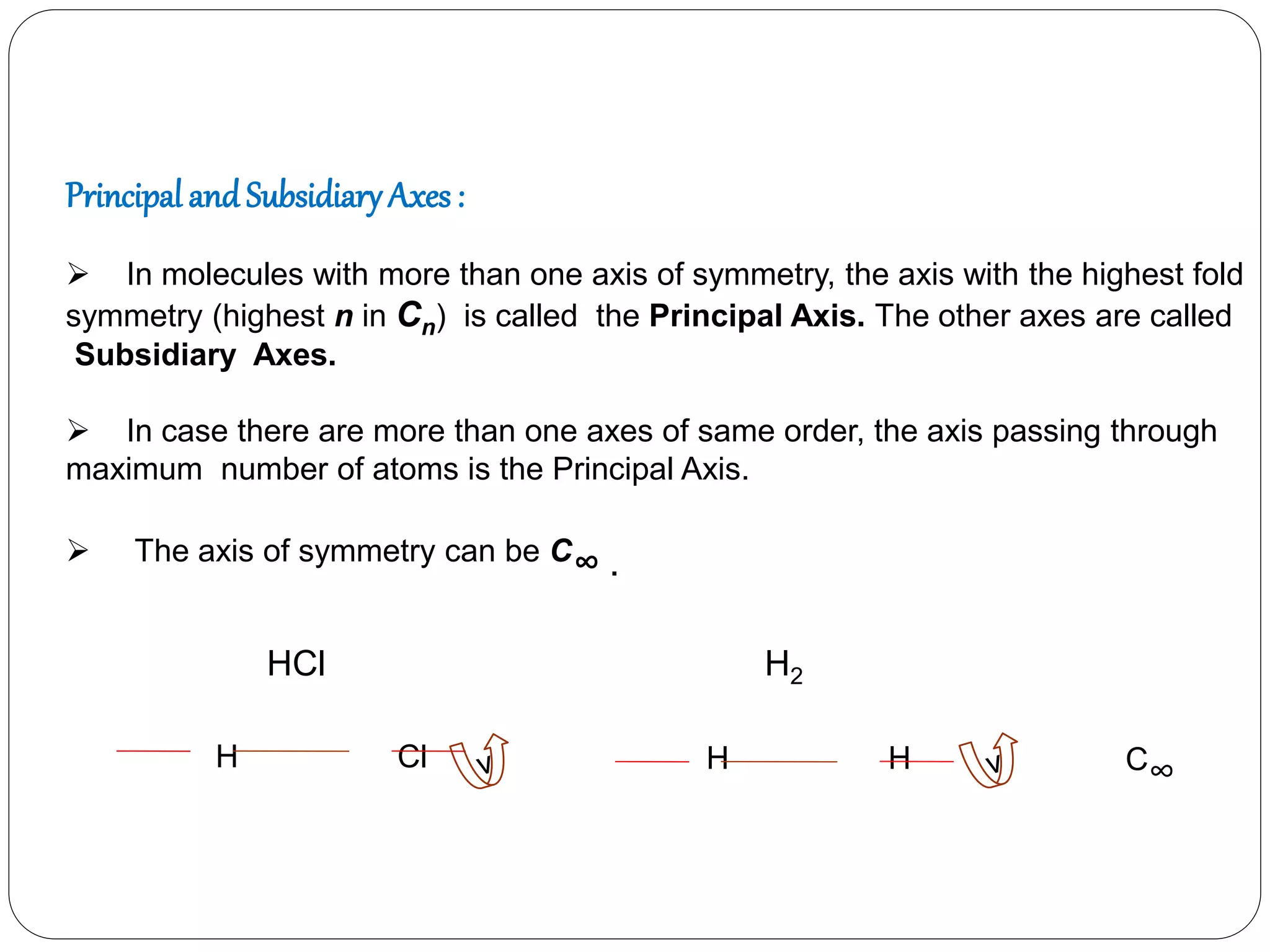
![3. Plane of symmetry [] :-
A mirror plane is an imaginary plane which
divides a molecules into two equal halves such
that one half is the exact mirror image of the
other.
It is denoted by ‘’.
Atoms on the surface of plane remain unshifted
during reflection.
Classification of mirror planes:-
Vertical plane(v) :- The principal axis of symmetry lies
in the this plane.
Horizontal plane (h):- The principal axis of symmetry
is perpendicular to the plane.
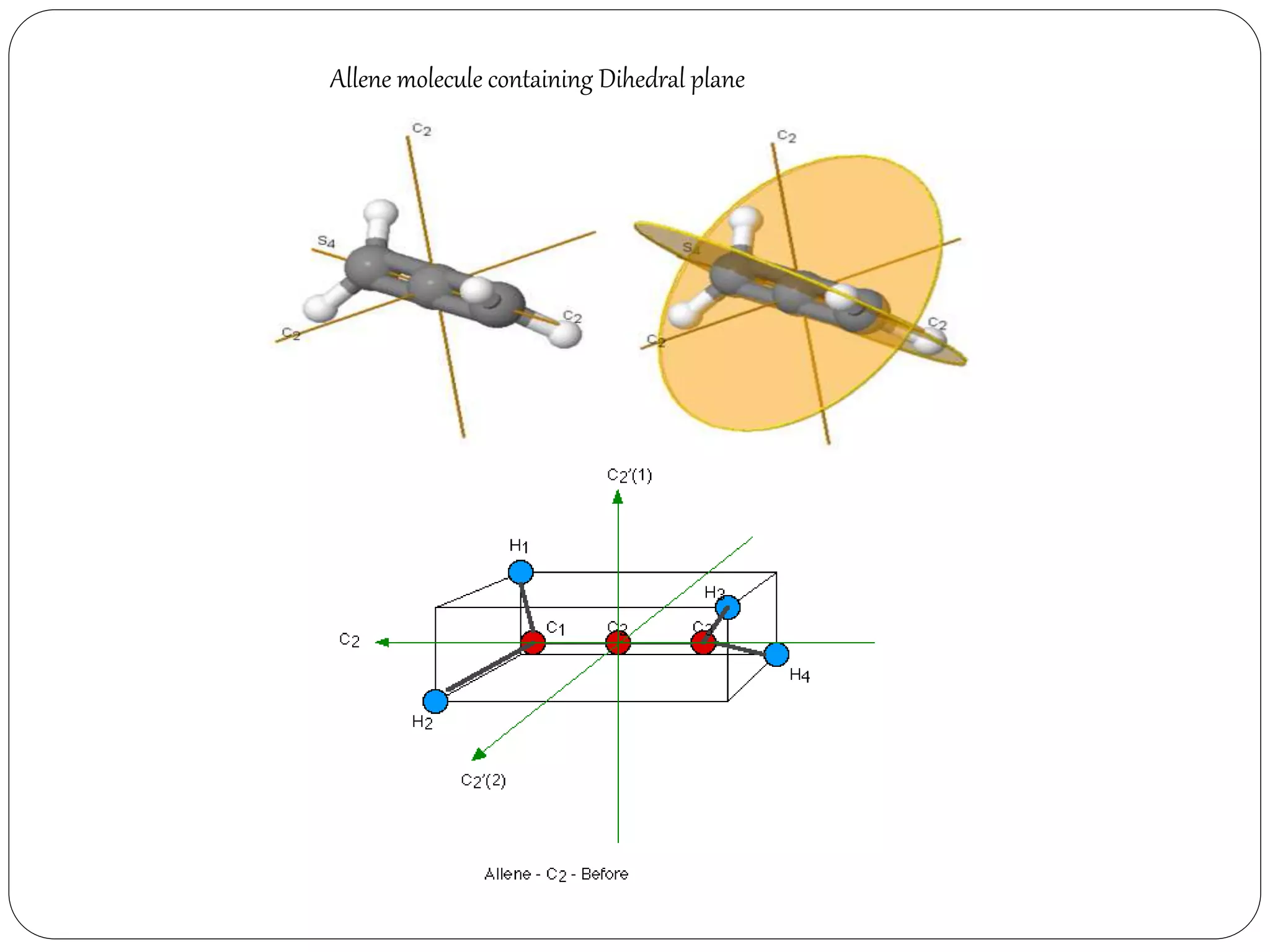
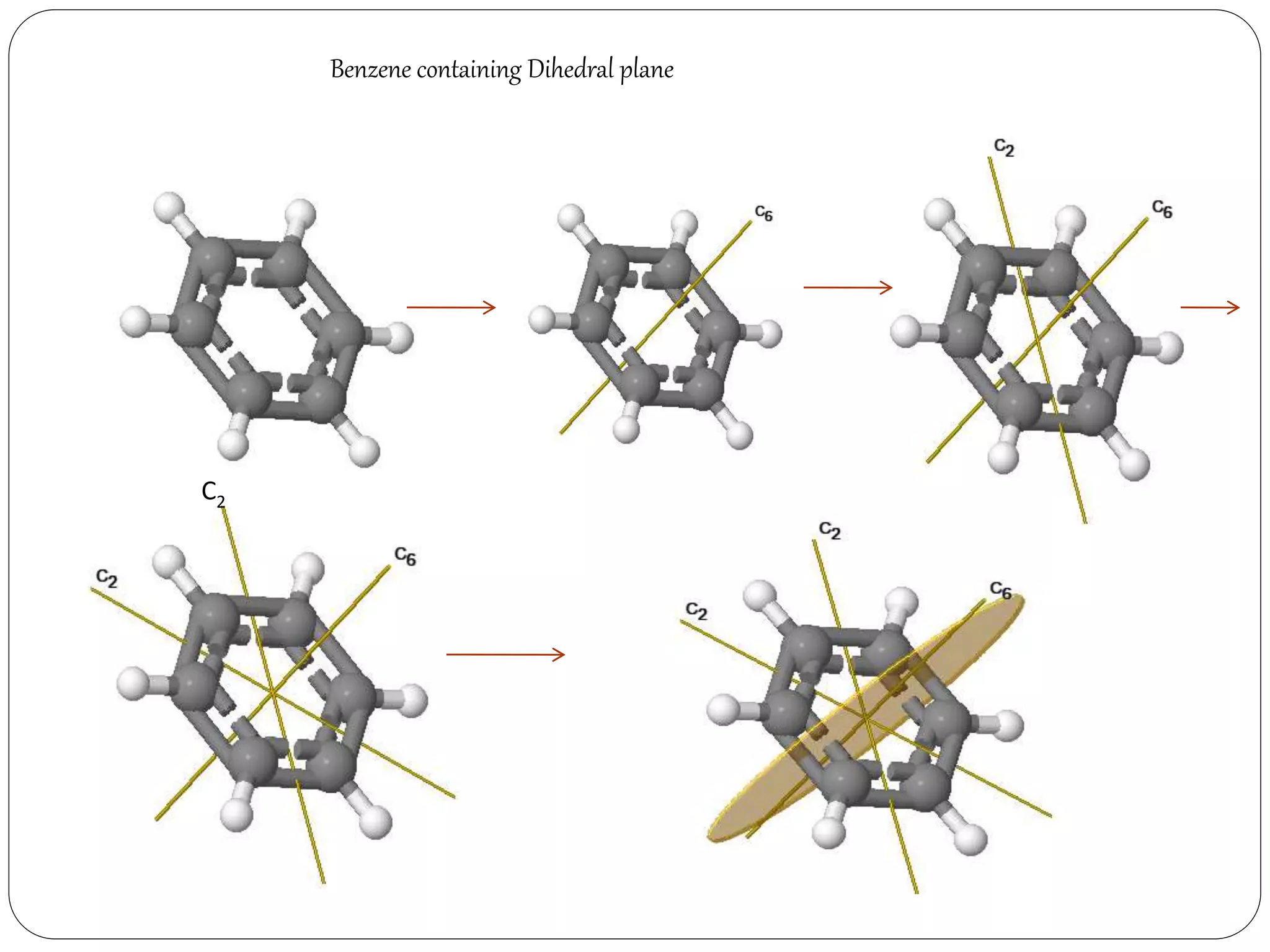
Dihedral plane (d):- The plane passing through the
principal axis but passing in between two subsidiary
axis, is the dihedral plane.](https://image.slidesharecdn.com/presentation1-170330052820/75/SYMMETRY-ELEMENTS-AND-SYMMETRY-OPERATIONS-23-2048.jpg)