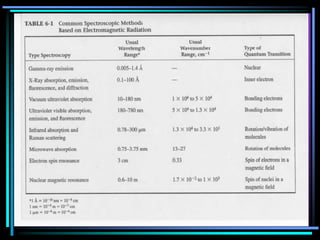
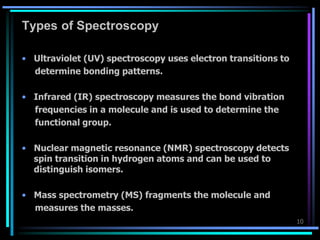
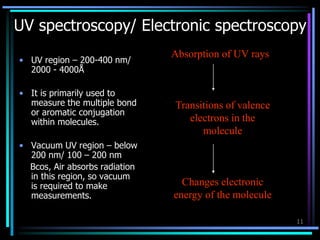
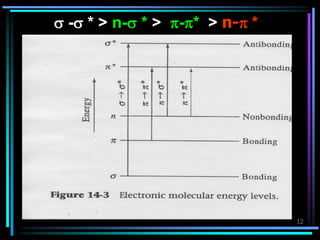
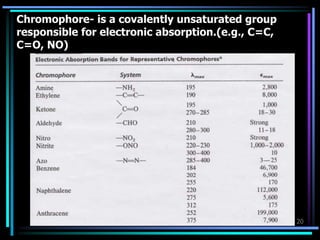
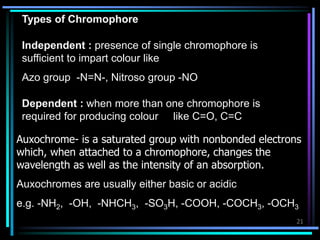
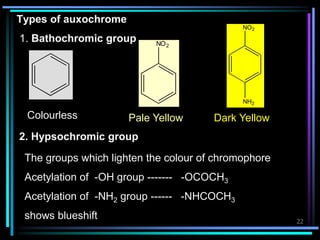
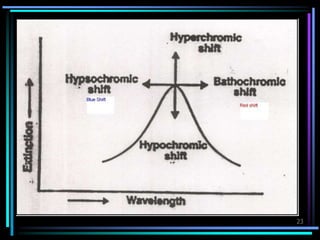
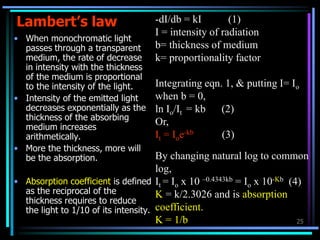
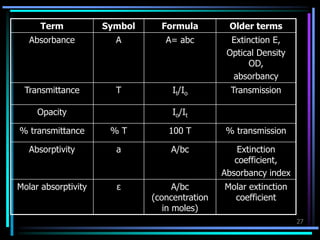
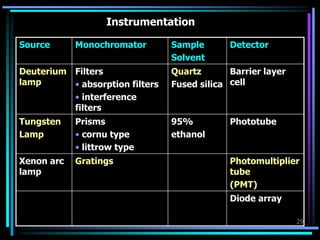
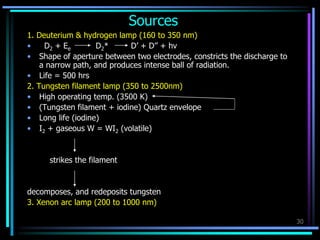
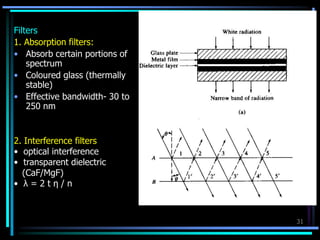
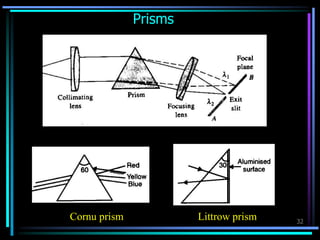
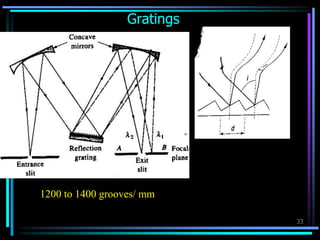
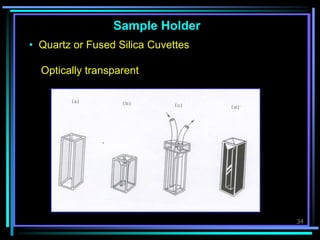
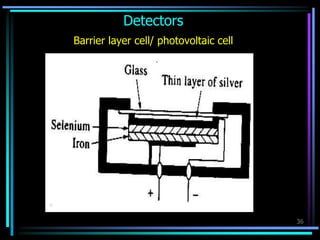
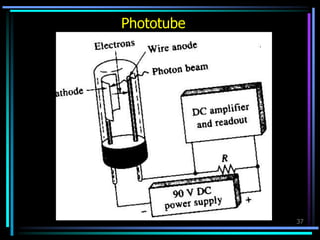
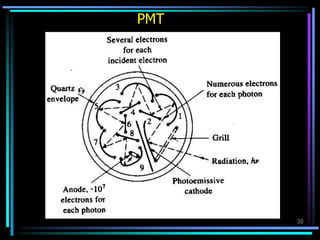
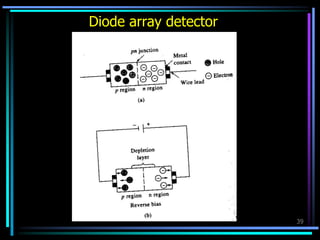
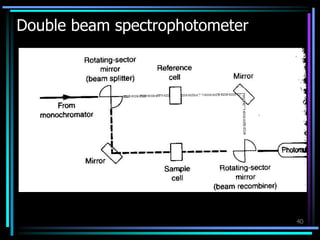
Ultraviolet (UV) spectroscopy uses absorption of UV light by molecules to determine their structure. It is based on electronic transitions in molecules that are excited by UV light. The document discusses UV spectroscopy terminology including wavelength, frequency, and energy. It describes different types of electronic transitions that can occur like σ-σ*, n-π*, and π-π* and how conjugation affects transitions. Instrumentation for UV spectroscopy is also covered including light sources, filters, gratings, sample holders, detectors, and applications in qualitative and quantitative analysis.


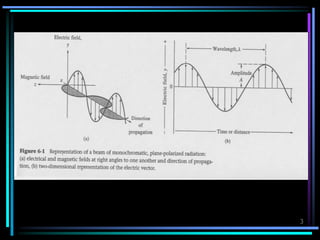
![7
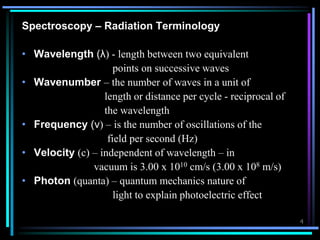
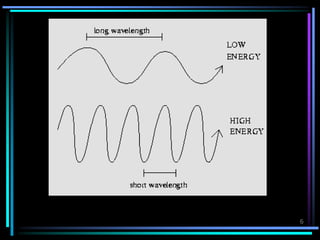
ν = c / λ -------------- [1]
• ν = frequency in Hz/cps
• c = 3 x 1010 cm/sec
• λ = wavelength in cm
E = hv --------------- [2]
• h= Planck’s constant (
• ν = frequency in Hz/cps
E = hc / λ --------------- [3]](https://image.slidesharecdn.com/ultravioletspectroscopy-190820113432/85/Ultra-violet-spectroscopy-7-320.jpg)