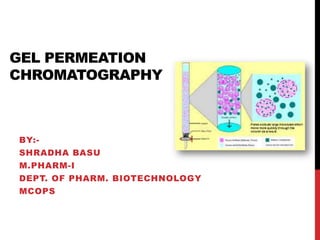
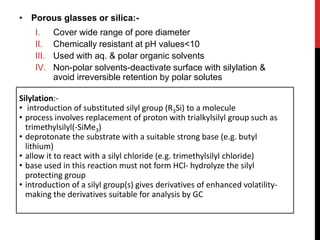
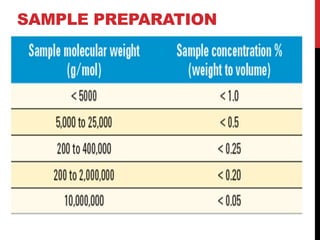
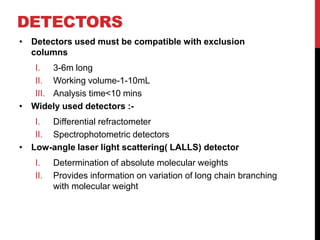
Gel permeation chromatography is a size-exclusion chromatography technique that separates analytes based on their hydrodynamic size. Molecules are separated by passing through a column containing porous gel or polymer beads. Small molecules are able to enter the pores and thus remain inside the beads longer, while larger molecules pass through more quickly. Detectors such as refractive index and UV-Vis are used to analyze the separated analyte bands exiting the column. GPC can determine molecular weight distribution and is used to analyze polymers, proteins, and other large biomolecules.