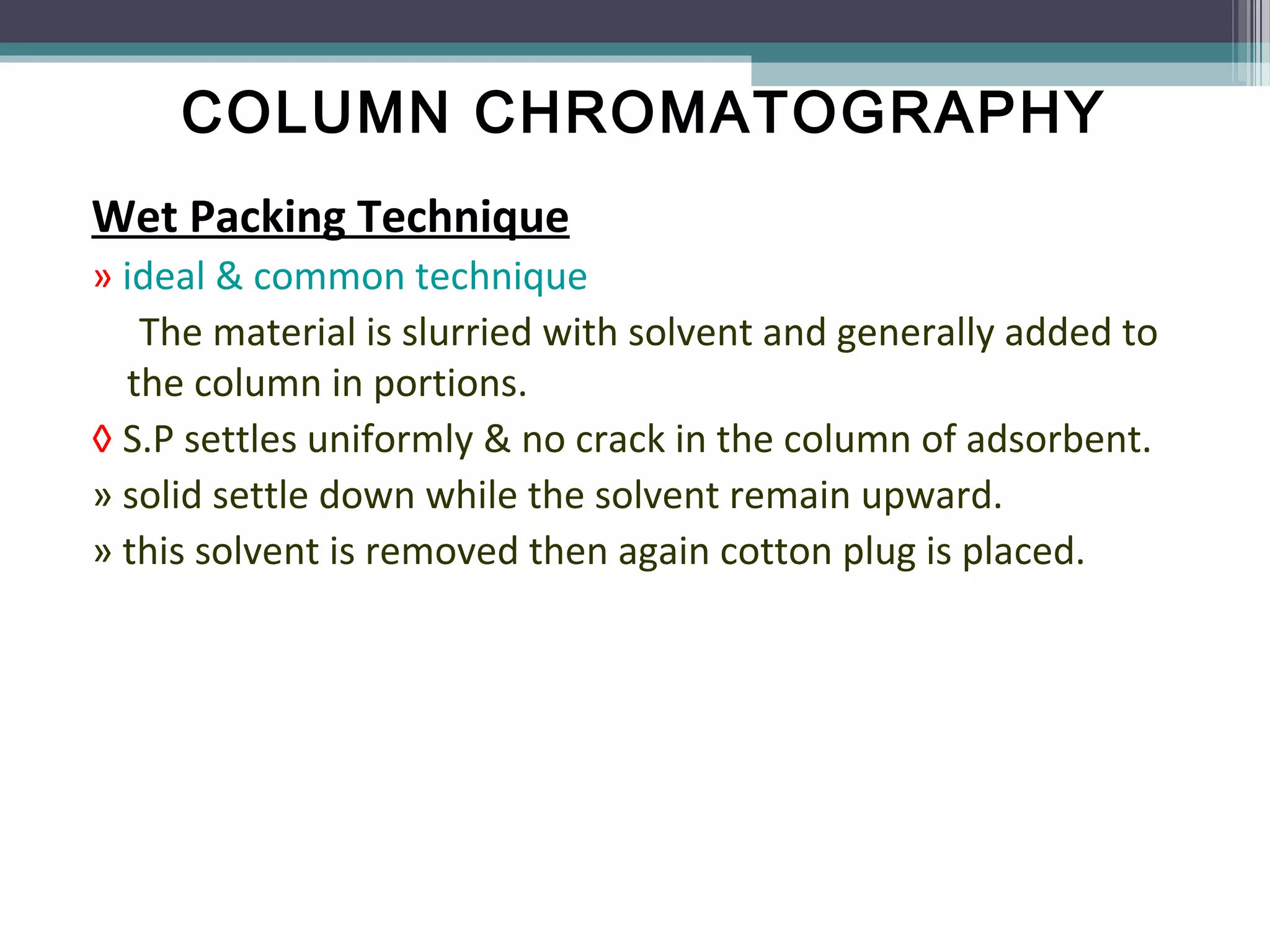
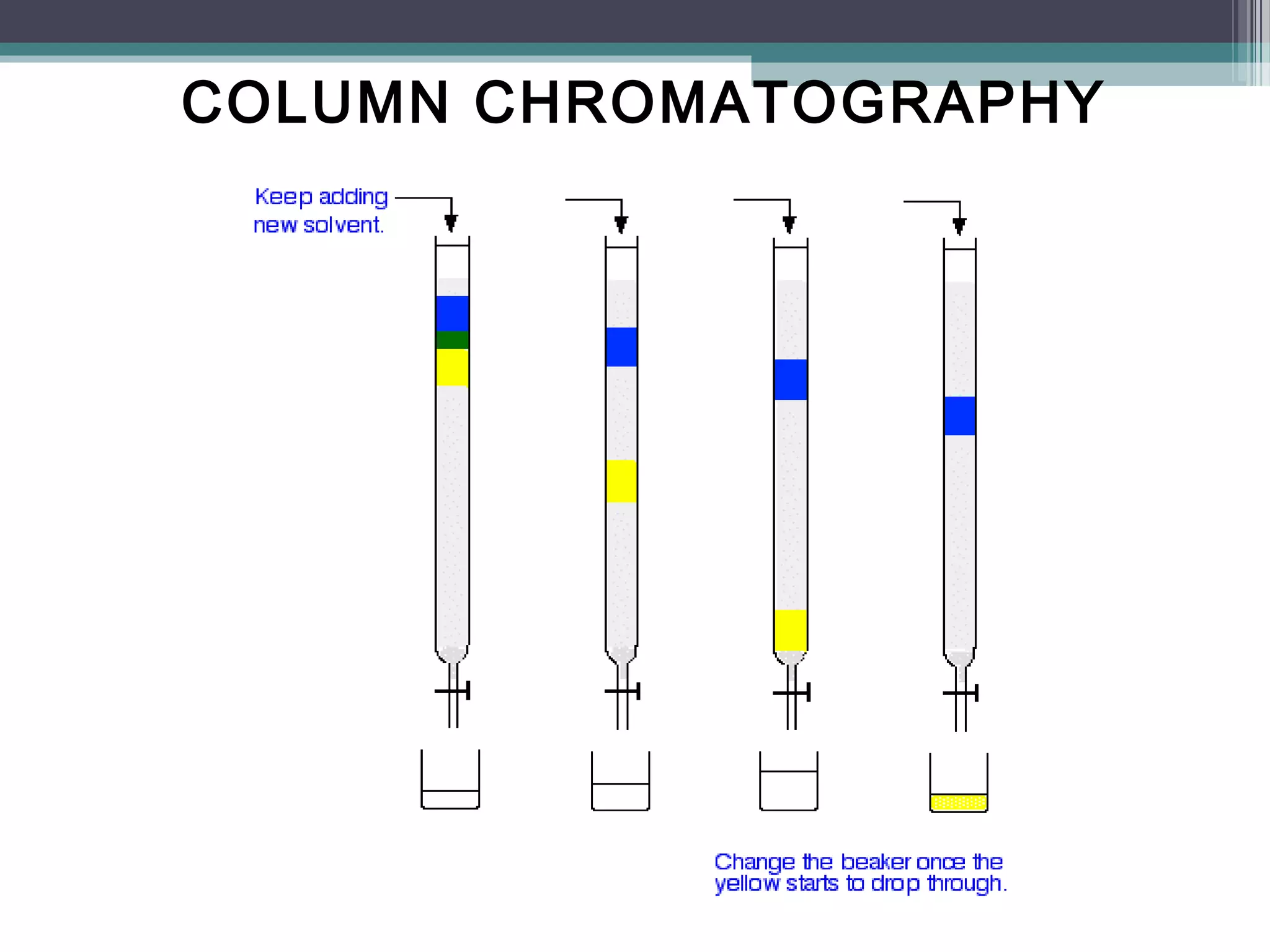
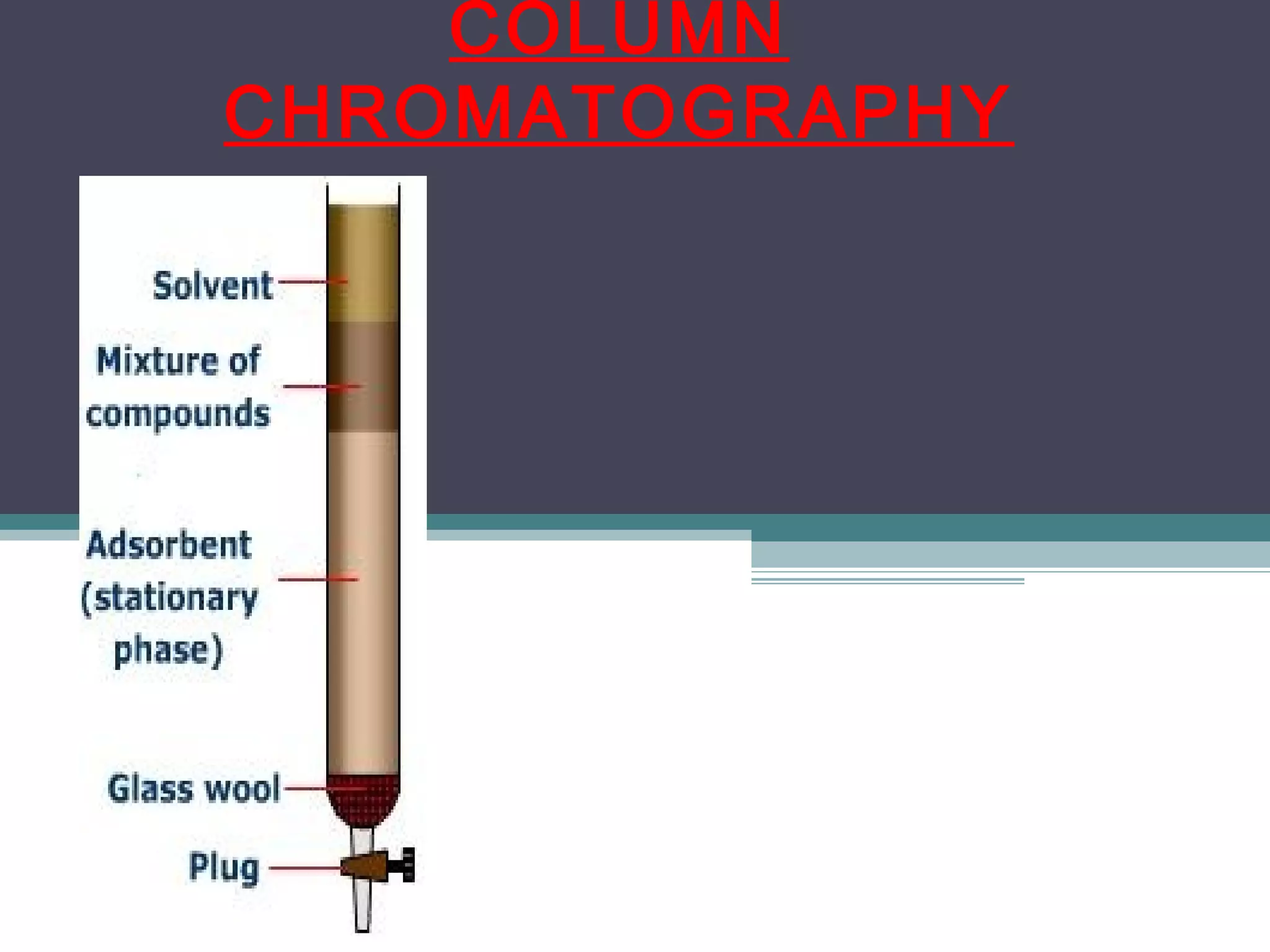
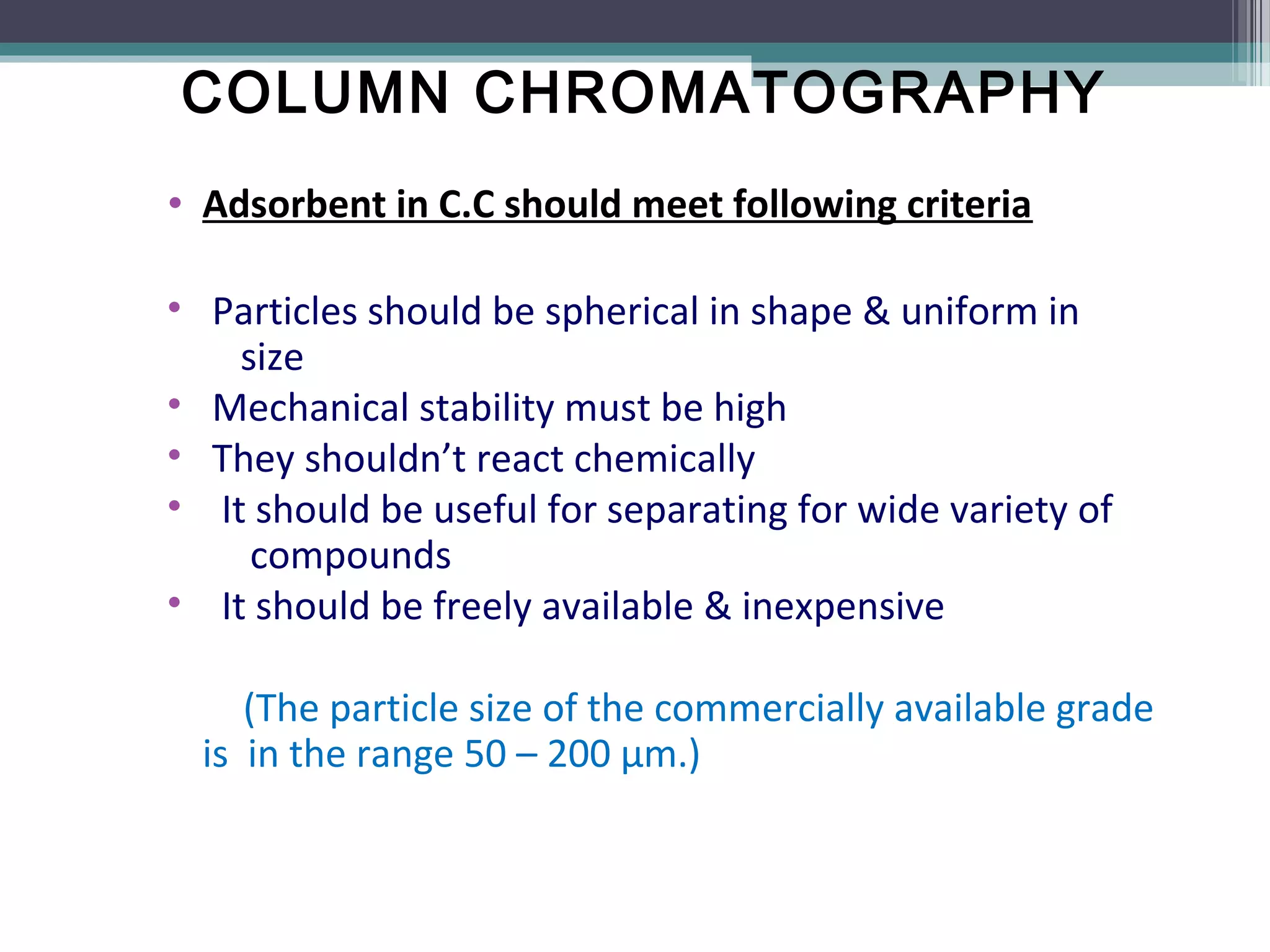
Column chromatography is a separation technique involving a mobile phase and a stationary phase to effectively separate and purify solids and liquids. It utilizes adsorption principles, with variations in mobile and stationary phases, packing techniques, and column types affecting its efficiency and application. Key factors in successful separation include the choice of adsorbent, mobile phase characteristics, and column dimensions.











![Types of Column
• 4-Vacuum Columns [Vacuum liquid
chromatography (VLC)]:
The adsorbent is applied dry into a sintered glass
funnel. The sample is applied by dry method or as
solution. Then the mobile phase is added portion by
portion and vacuum is applied after each portion to
collect each fraction.](https://image.slidesharecdn.com/column-chromatography-ppt-190210075647/75/Column-chromatography-14-2048.jpg)