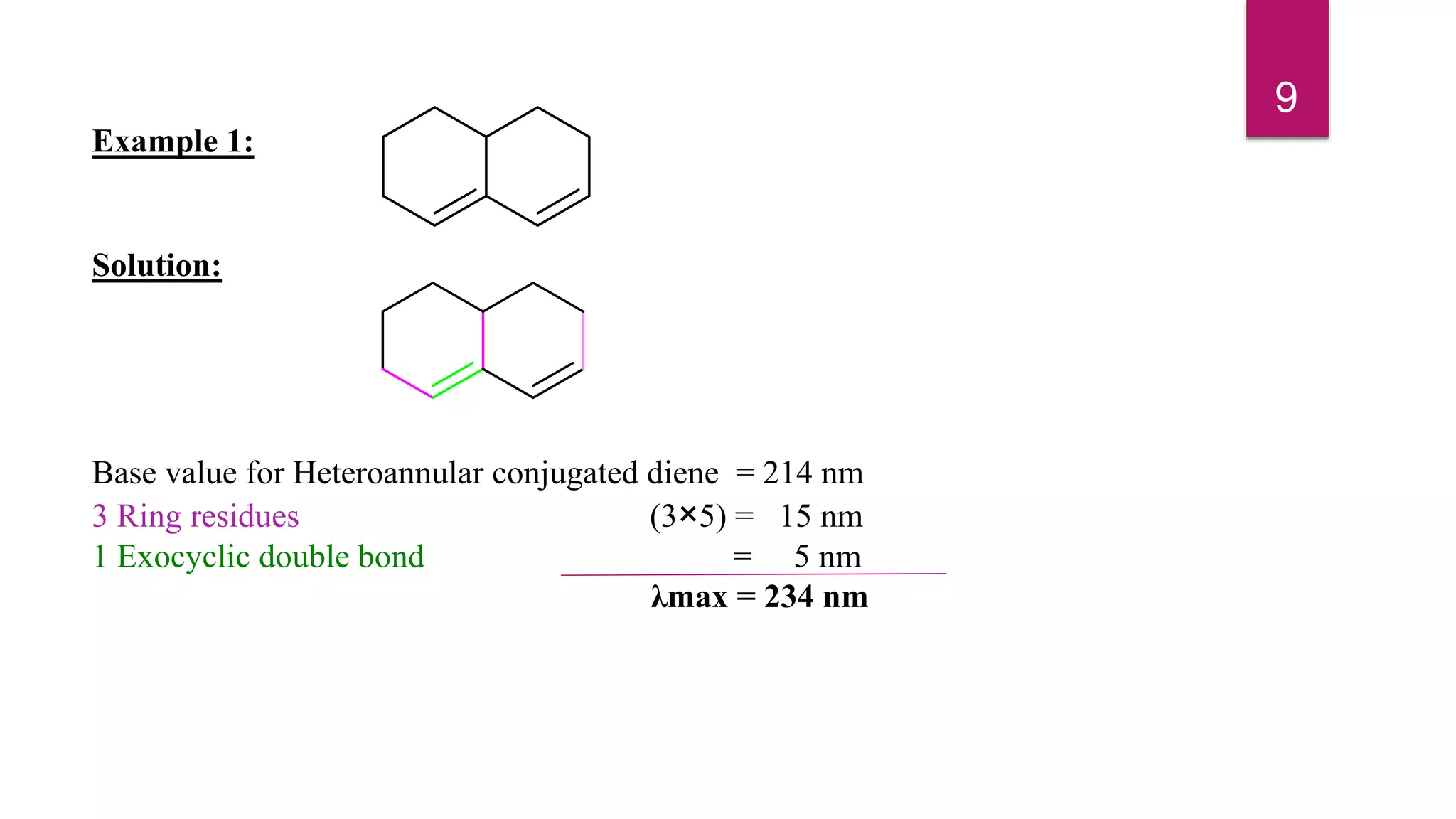
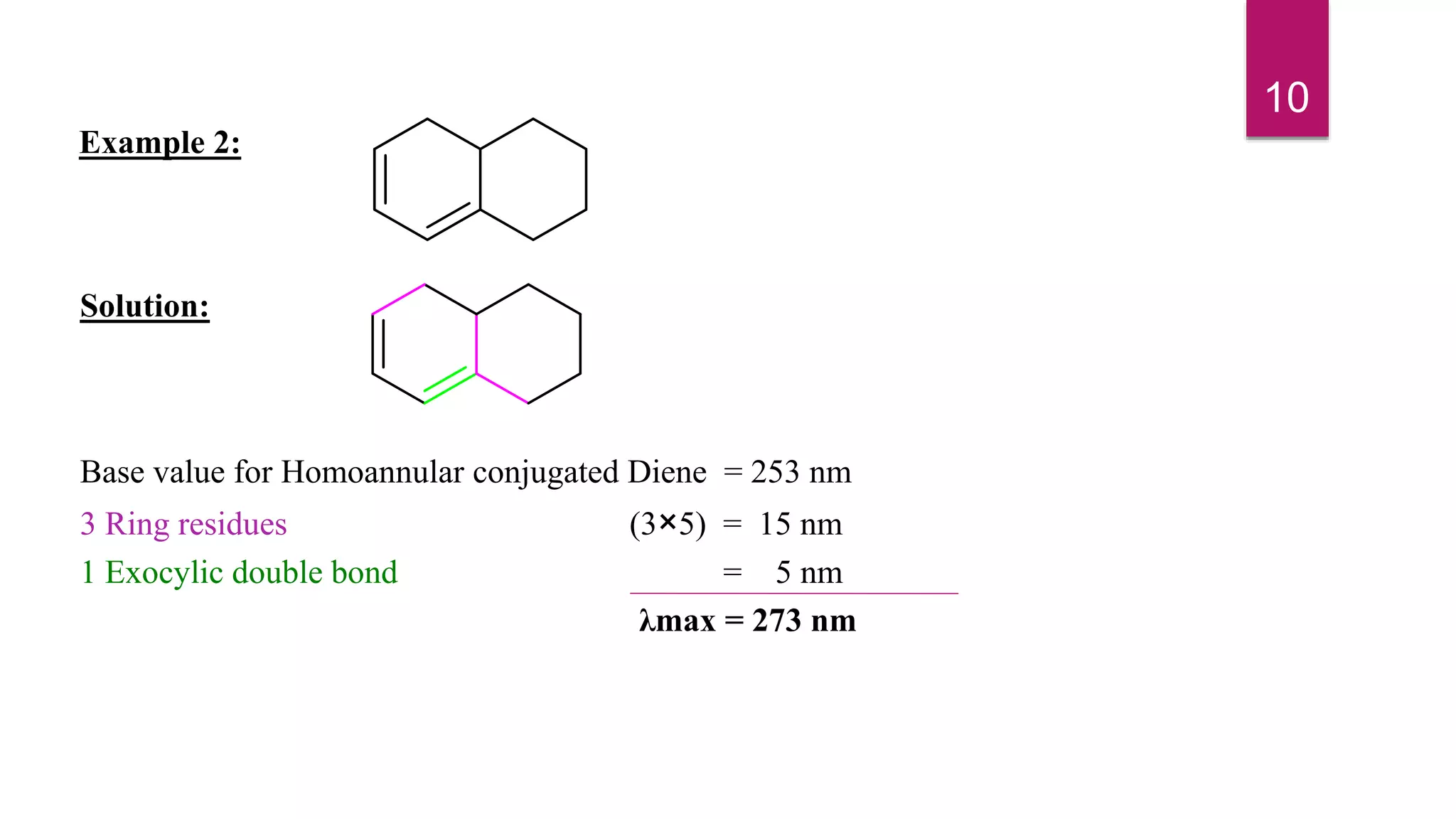
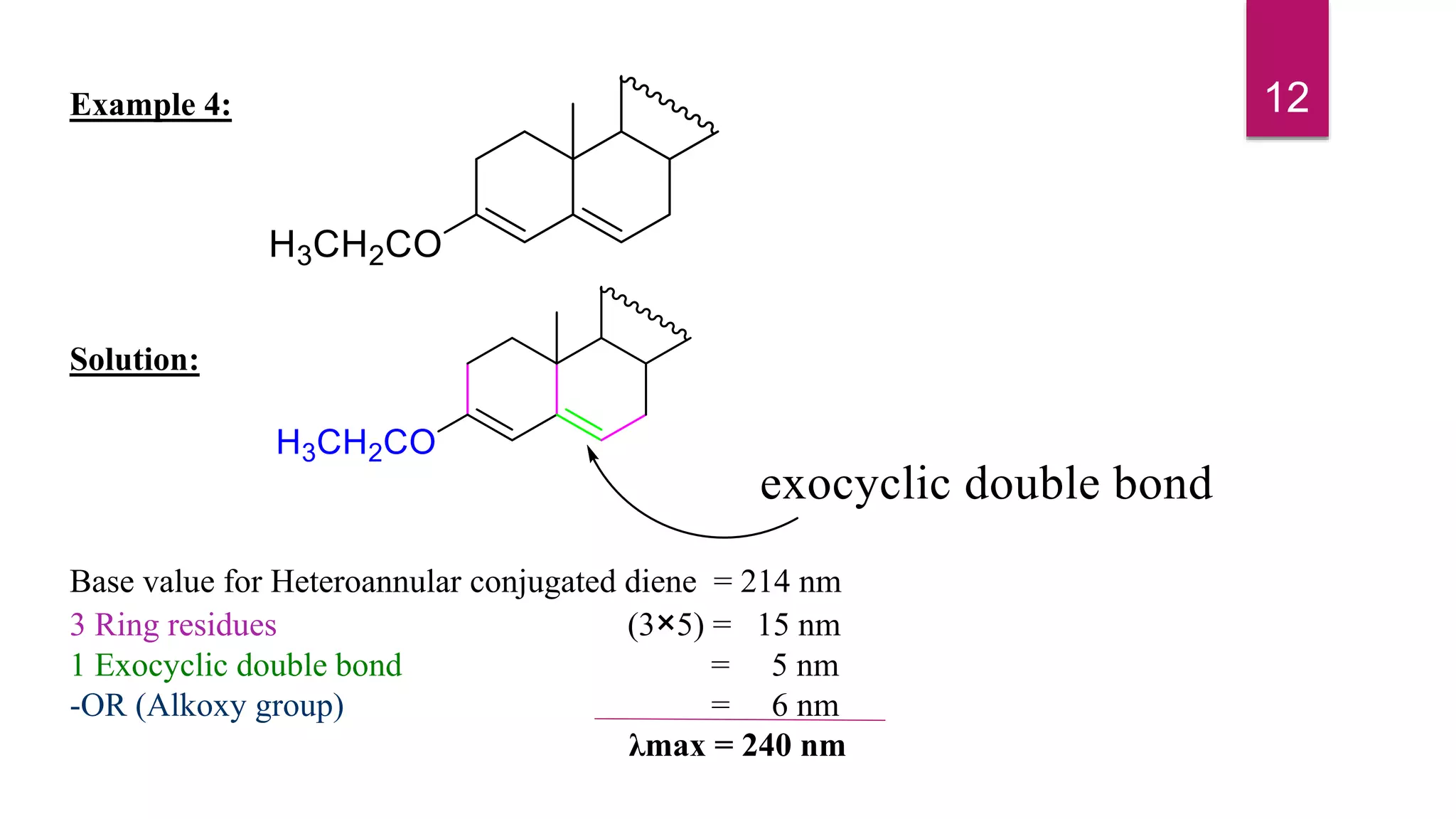
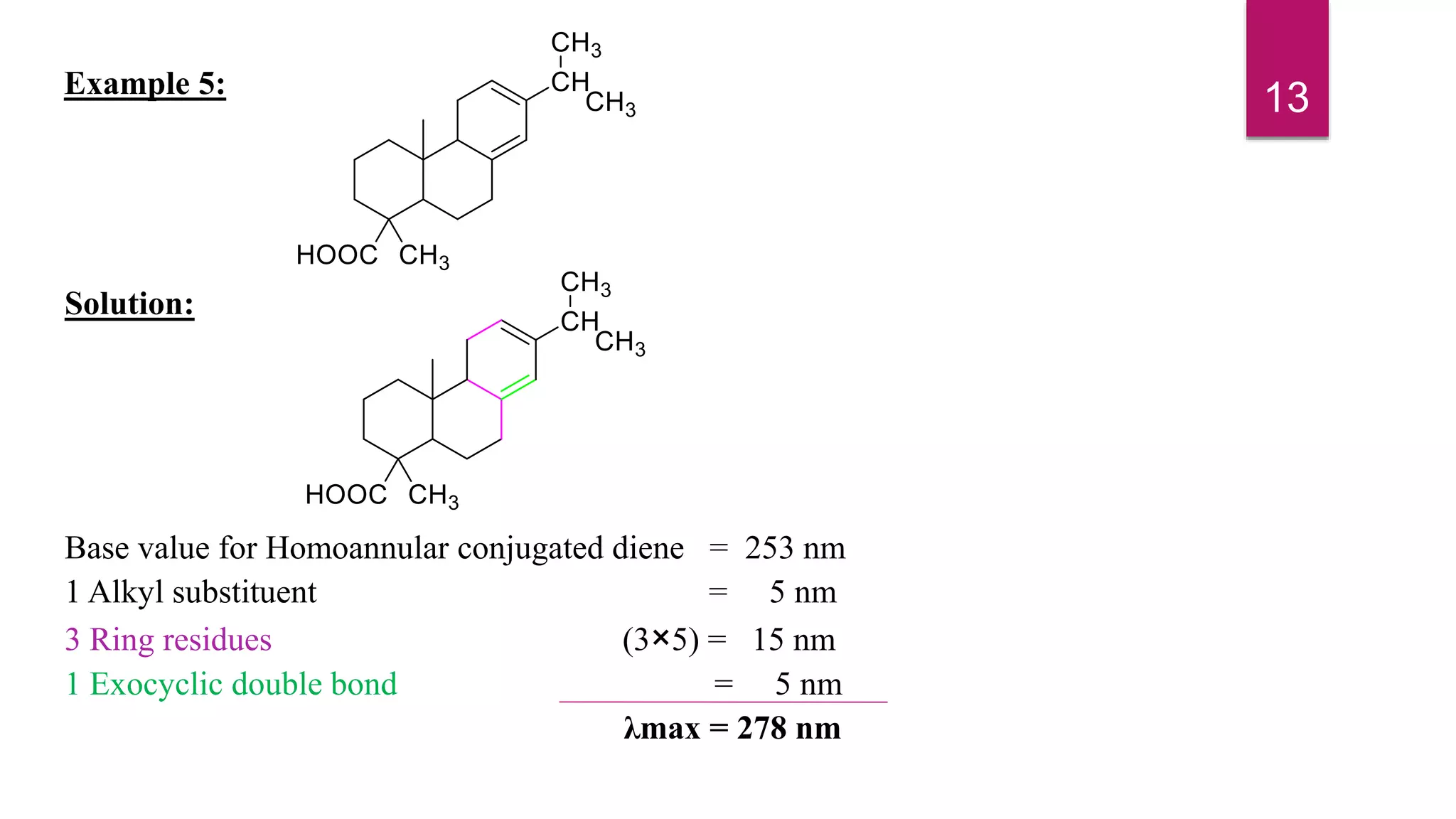
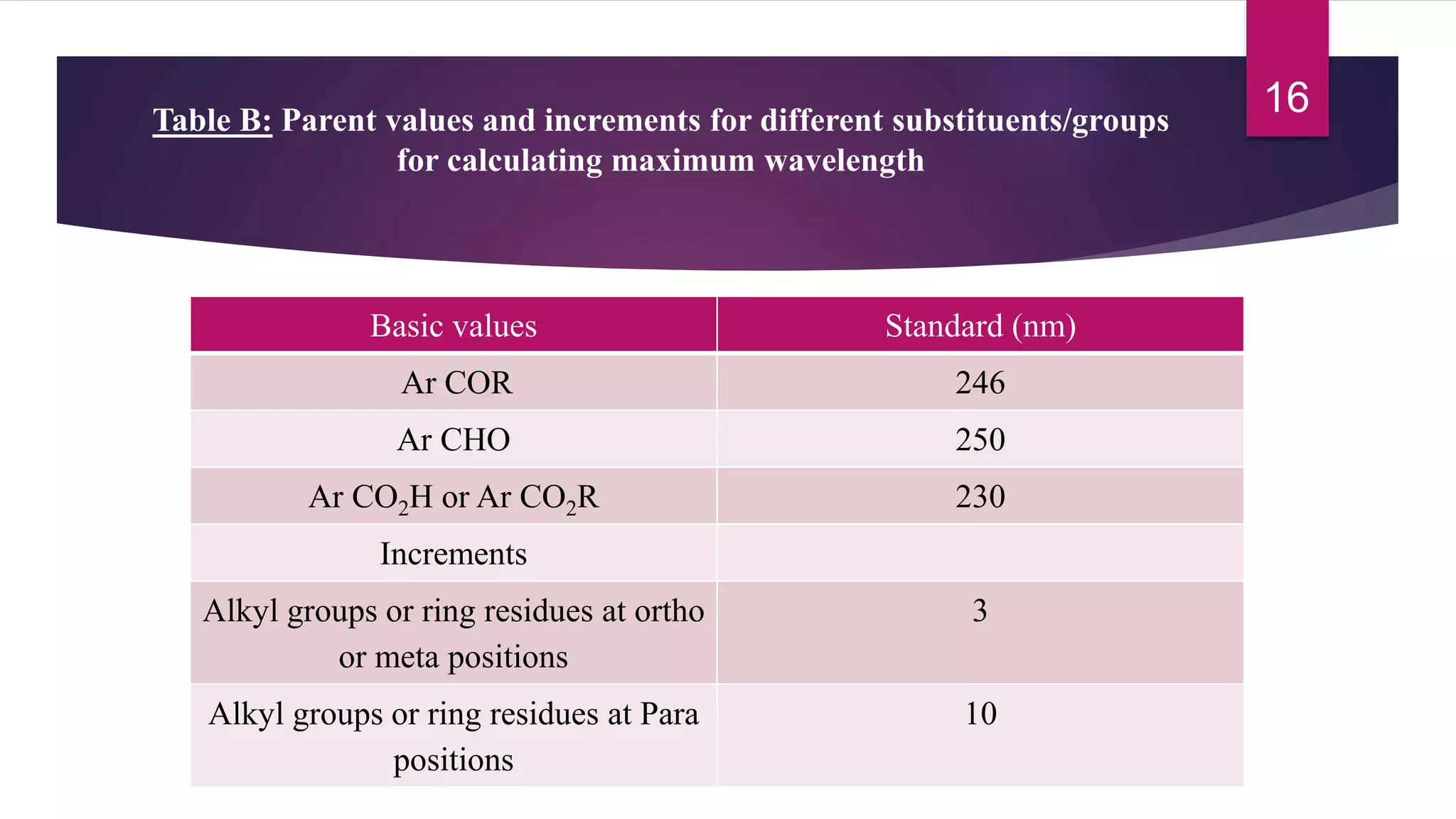
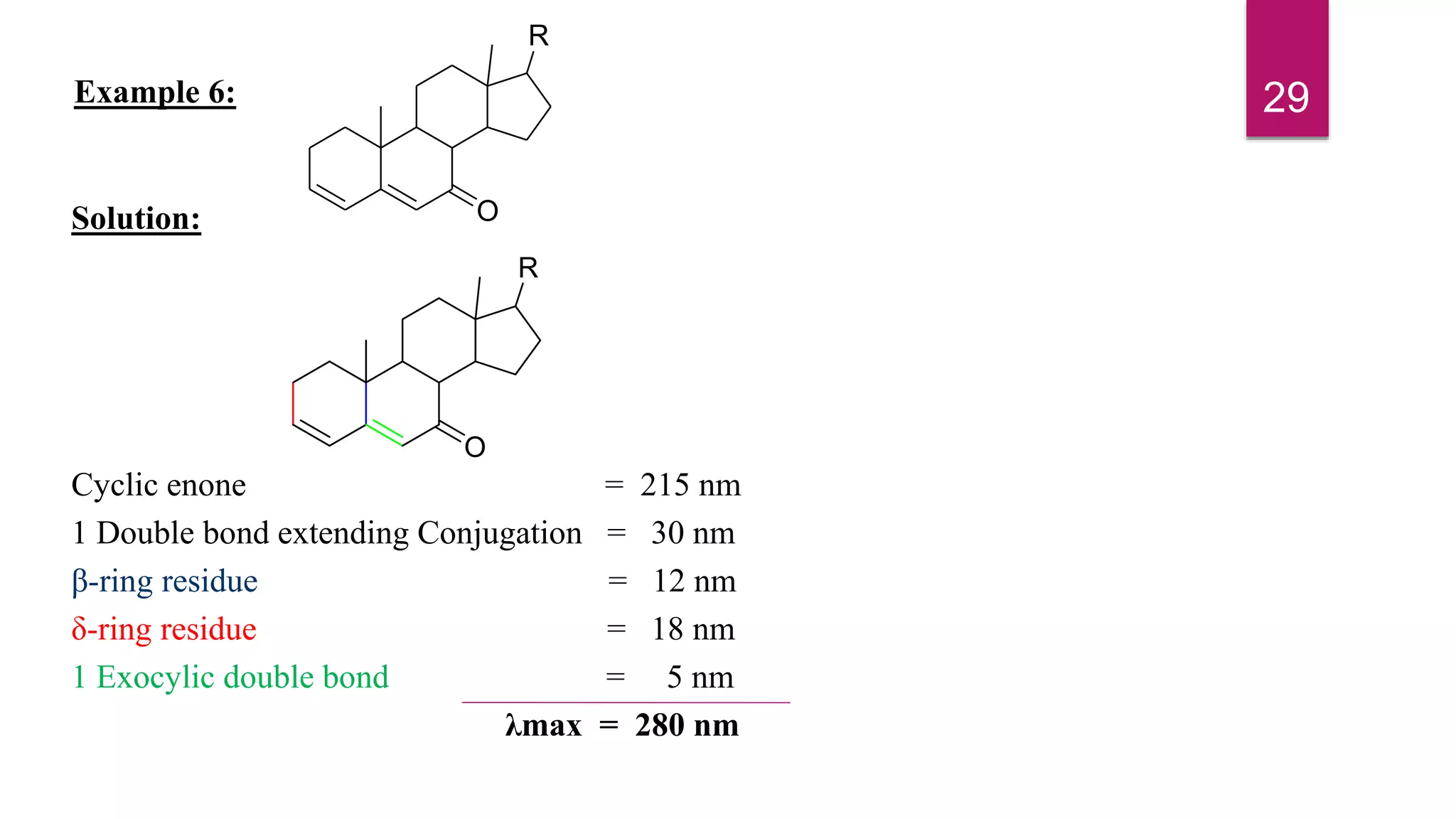
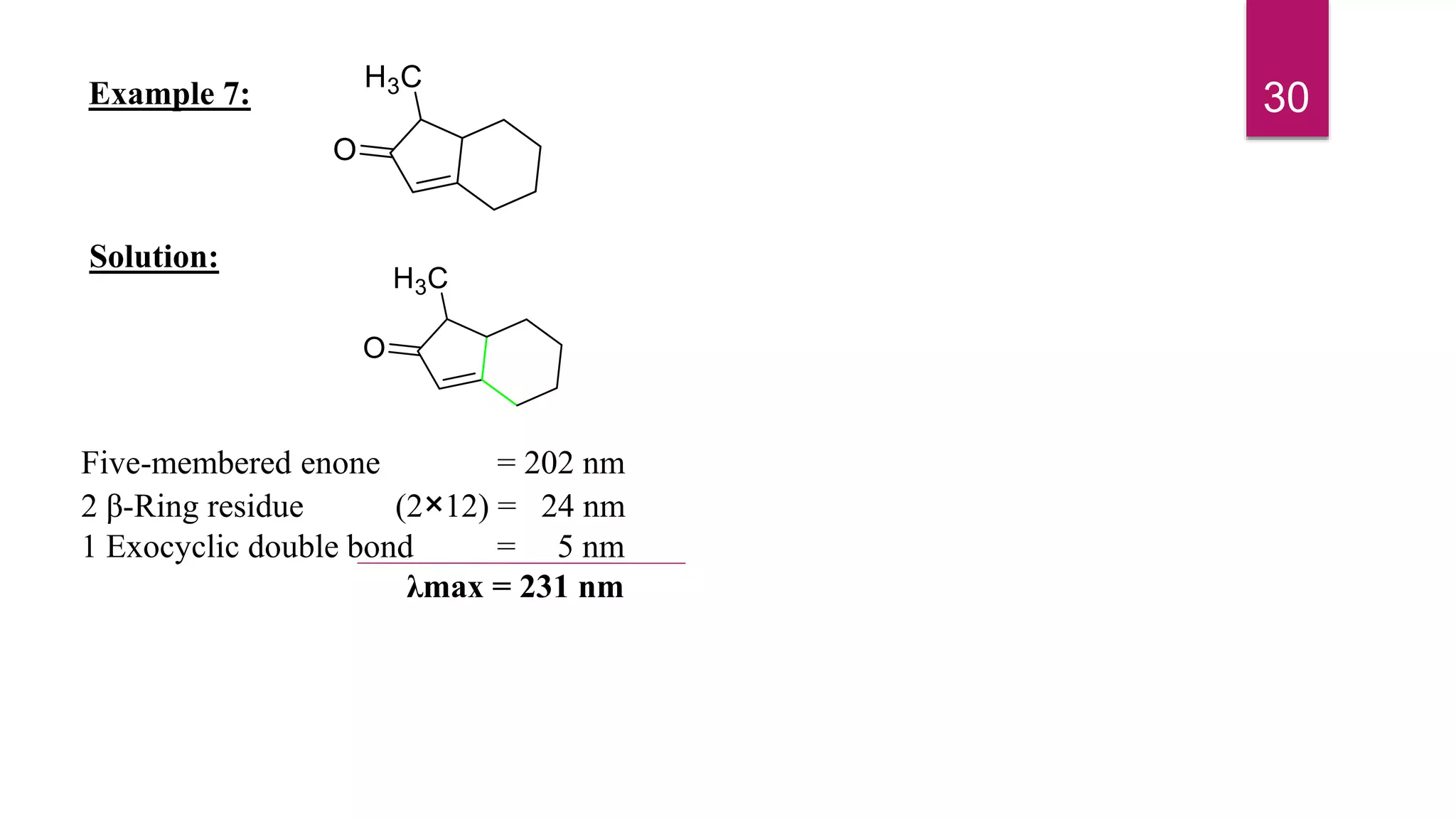
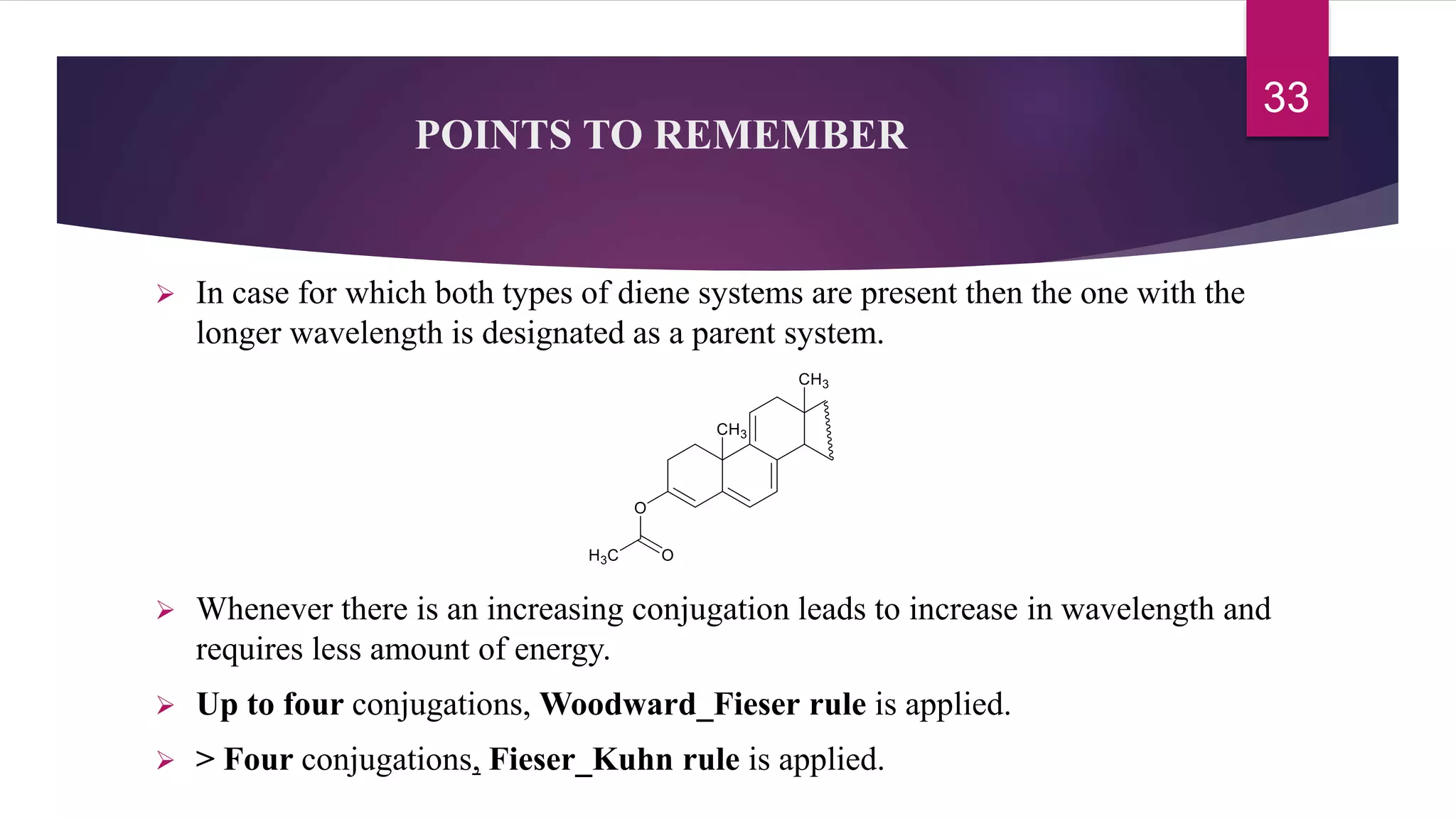
This document discusses the Woodward-Fieser rules for predicting the wavelength of maximum absorption (λmax) of organic compounds based on their molecular structure. It introduces the basic terminology and presents the parent values and incremental contributions for calculating λmax for different functional groups in conjugated dienes, aromatics, α,β-unsaturated carbonyls, and compounds with more than four conjugated double bonds. Examples are provided to demonstrate the application of the rules for each class of compounds. The document is intended as an introduction to the Woodward-Fieser rules and their use in predicting UV-vis absorption spectra based on molecular structure.