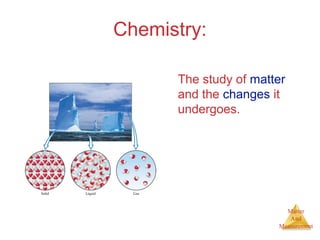
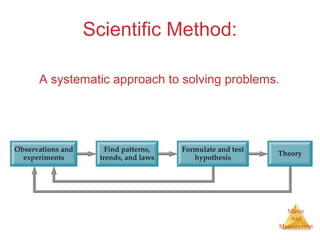
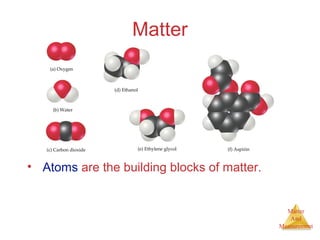
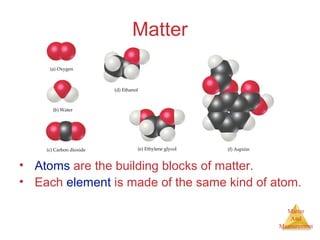
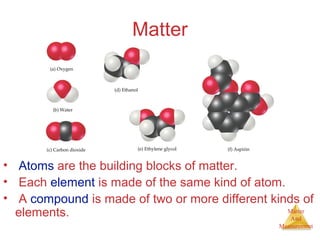
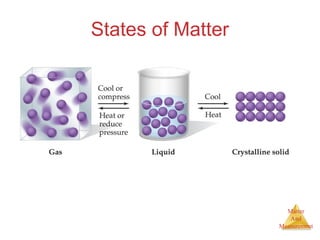
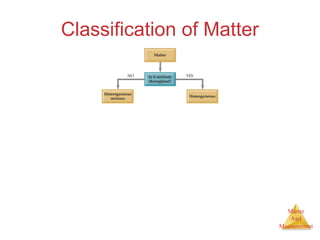
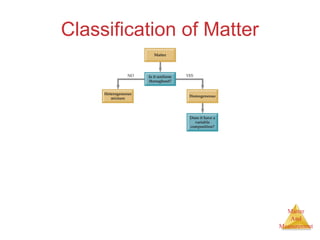
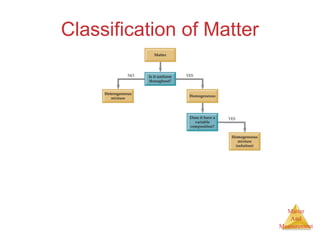
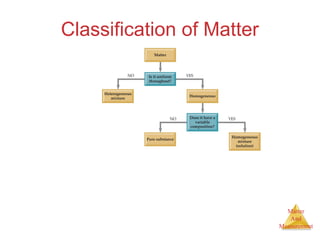
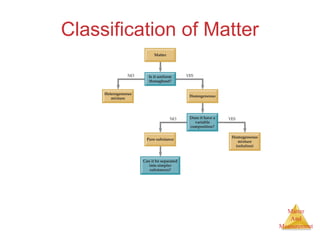
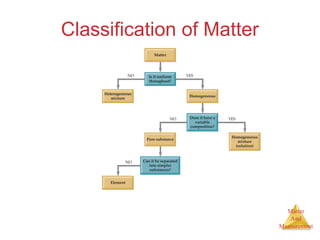
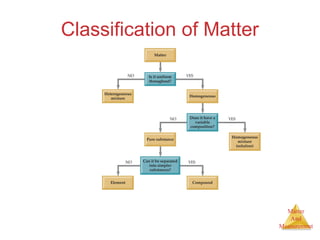
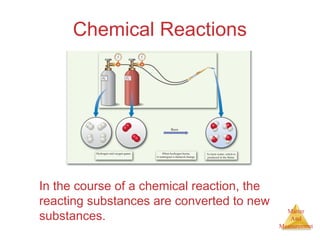
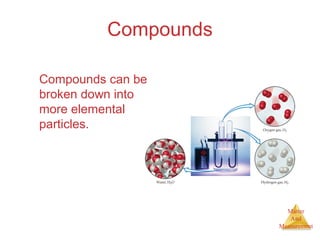
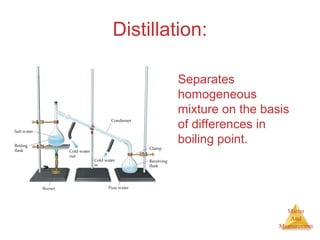
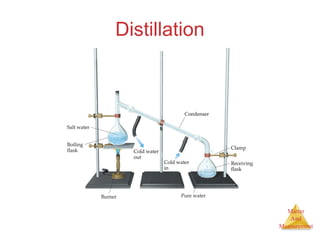
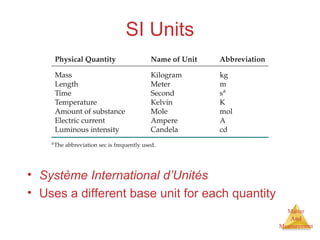
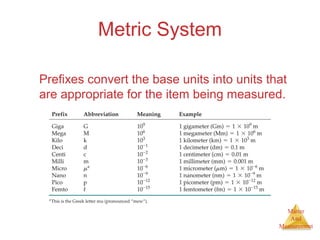
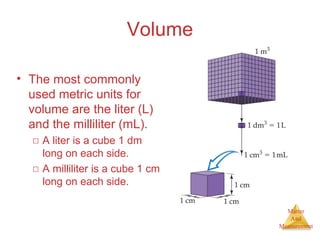
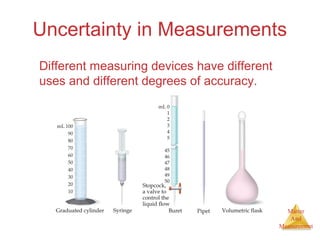
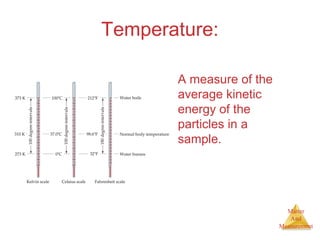
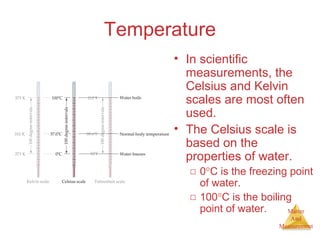
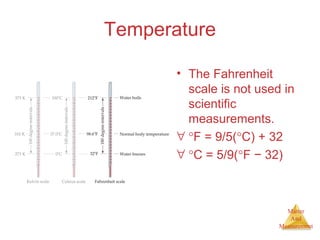
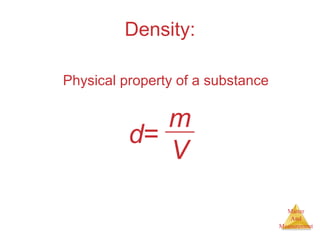
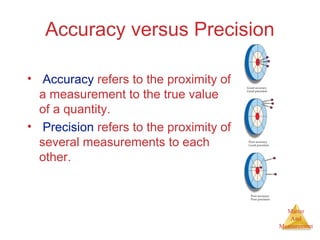
This document provides an overview of matter and measurement in chemistry. It defines chemistry as the study of matter and changes in matter. Matter is defined as anything that has mass and takes up space, and is composed of atoms as building blocks. Compounds are made of two or more different types of elements. The document also discusses states of matter, properties and changes in matter, units of measurement in the International System of Units (SI), and significant figures in measurements and calculations.