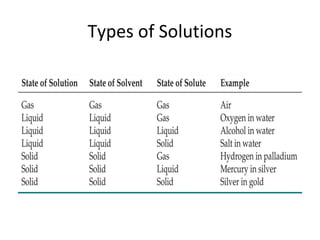
This document provides information on solution chemistry and concepts including:
- Definitions of key terms like solution, solute, and solvent
- The process of dissolution where solvent molecules pull apart solute molecules
- How saturated, supersaturated and concentrated solutions are classified
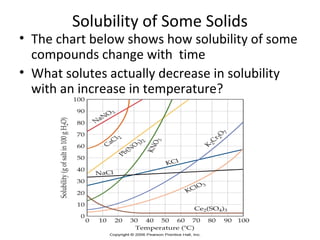
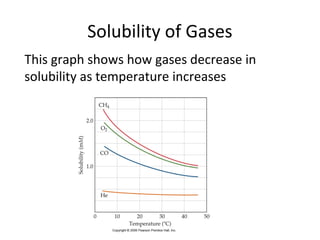
- Factors that influence solubility like temperature, pressure and nature of solute
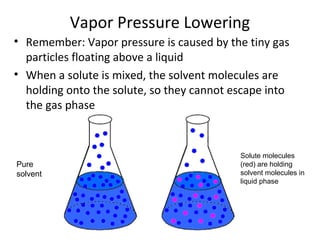
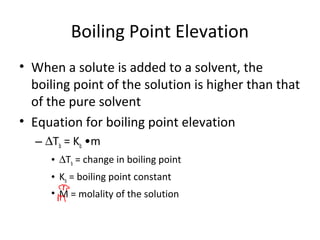
- Colligative properties of solutions like vapor pressure lowering, freezing point depression and boiling point elevation that depend on amount of solute.
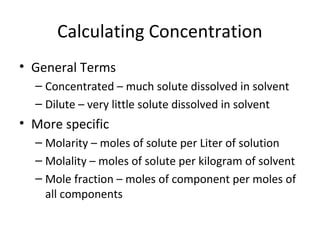
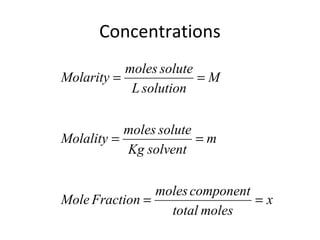
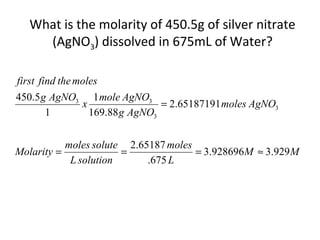
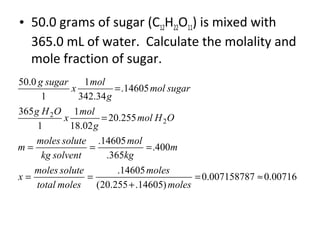
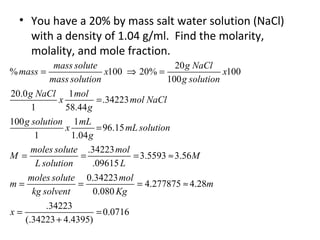
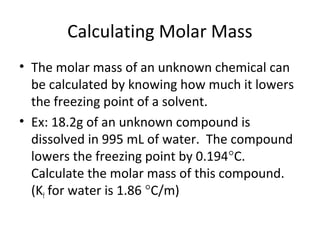
- Equations to calculate values like molarity, molality and mole fraction in solutions.