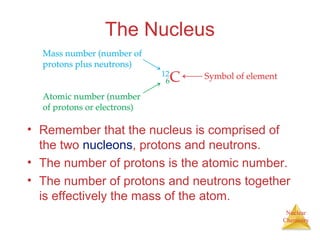
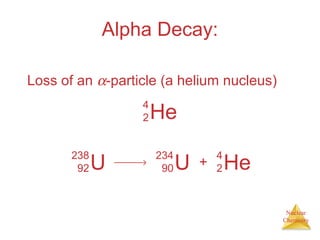
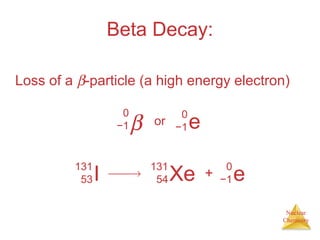
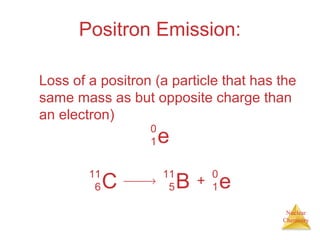
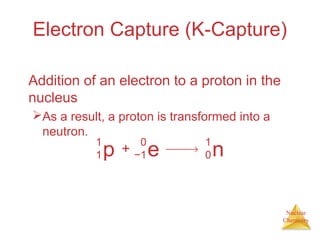
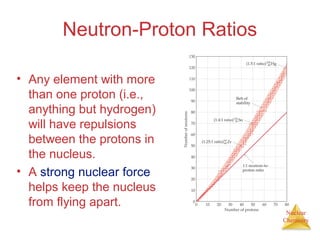
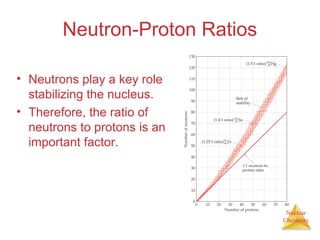
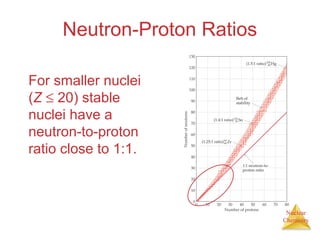
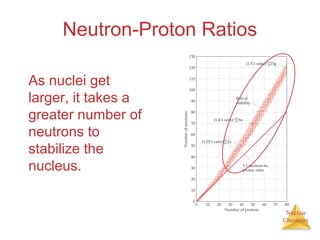
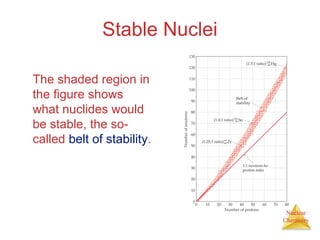
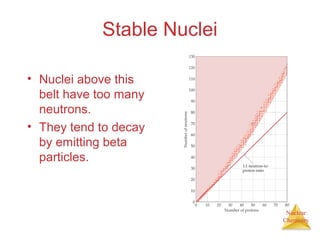
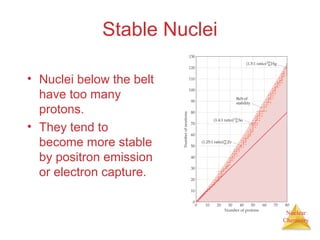
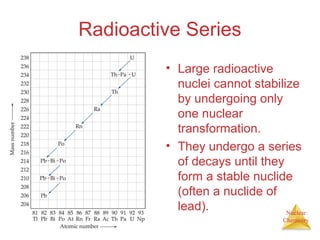
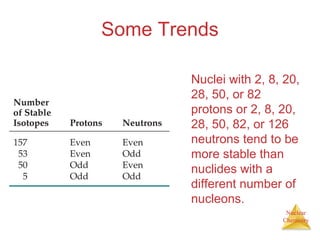
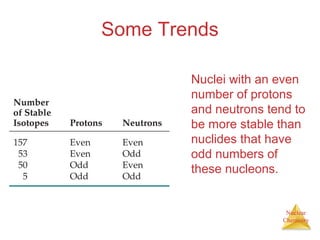
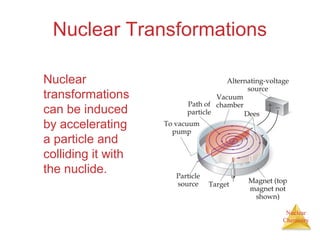
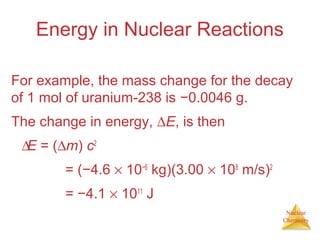
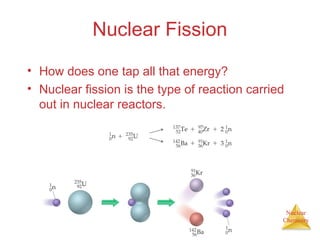
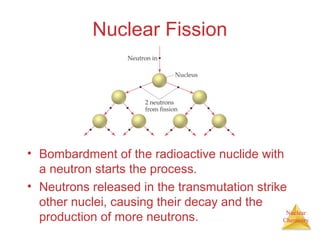
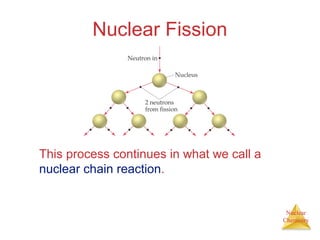
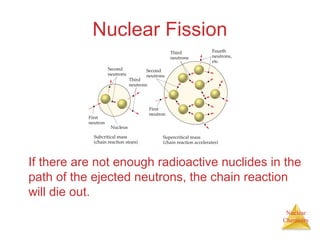
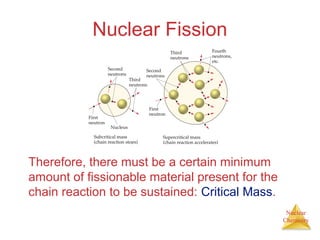
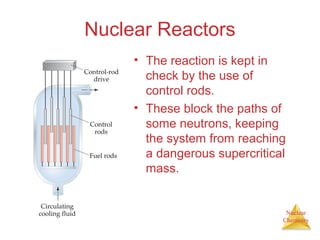
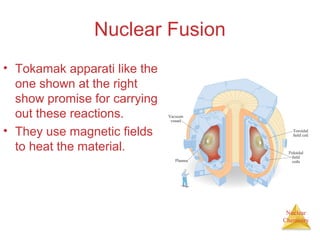
1) The nucleus is comprised of protons and neutrons, with the number of protons defining the element. 2) Isotopes of the same element have different numbers of neutrons, resulting in slightly different masses. 3) Some nuclei are unstable and undergo radioactive decay through processes like alpha, beta, or gamma emission to become more stable nuclides. 4) Nuclear reactions involve tremendous amounts of energy due to mass-energy equivalence, and can be harnessed through fission in reactors or potential fusion.