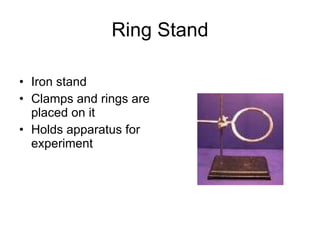
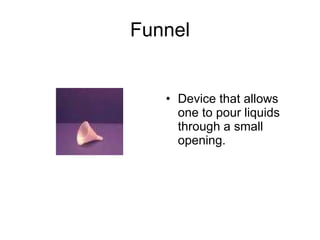
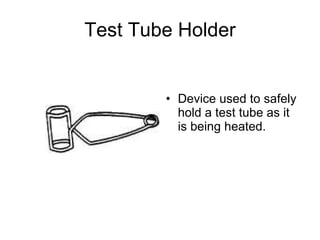
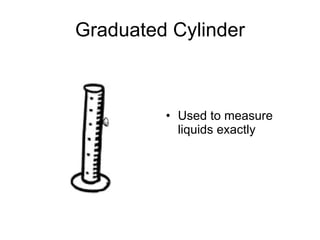
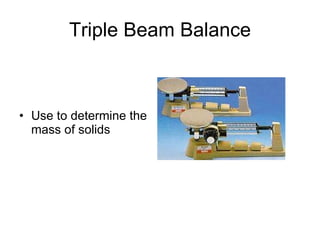
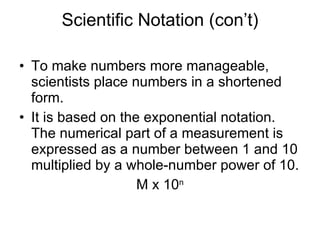
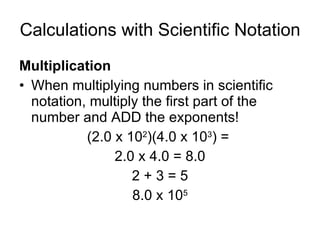
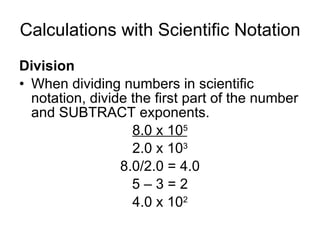
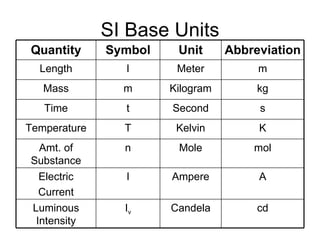
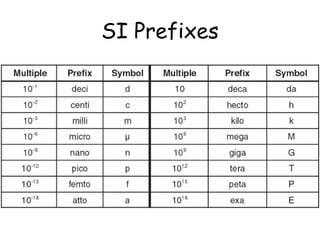
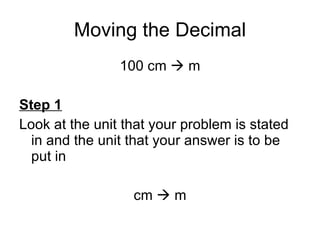
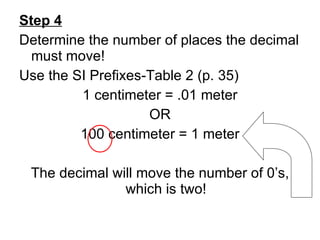
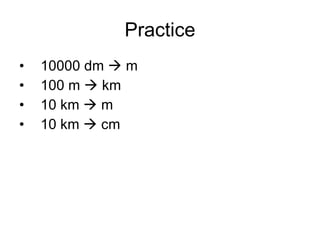
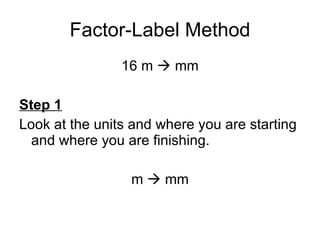
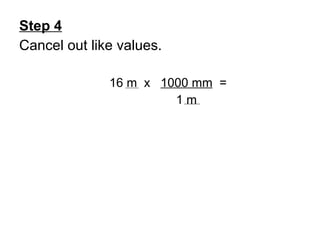
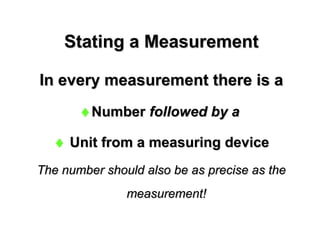
This document provides an overview of chemistry including:
- Chemistry is the study of matter, its structure and properties, and how matter changes.
- There are many careers in fields like engineering, medicine, and more that involve chemistry.
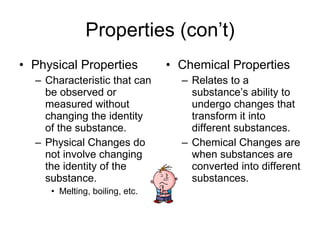
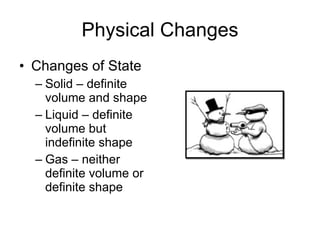
- Matter is made up of elements, compounds, and mixtures. Properties include physical properties that can be observed without changing identity, and chemical properties involving changes to form new substances.