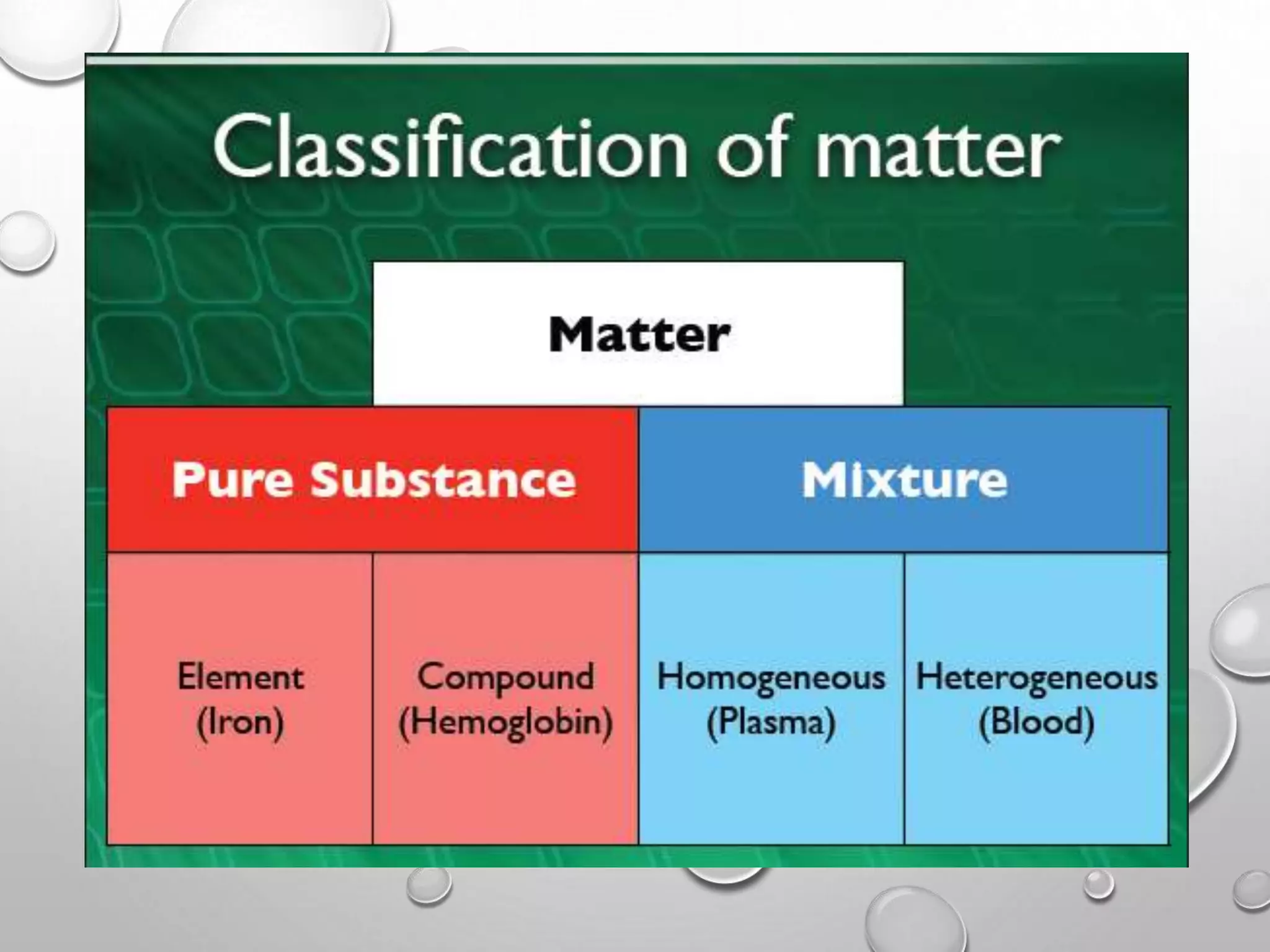
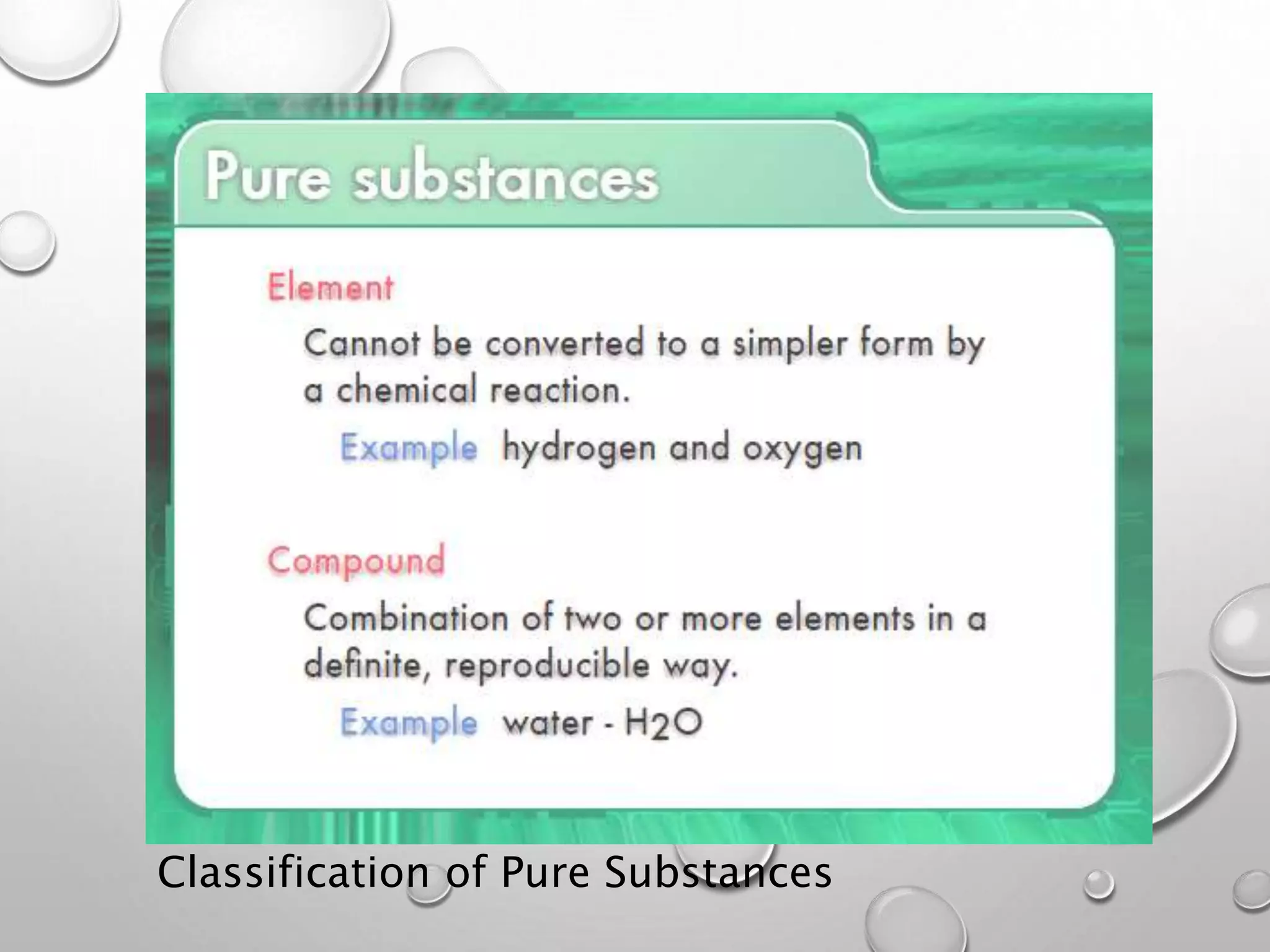
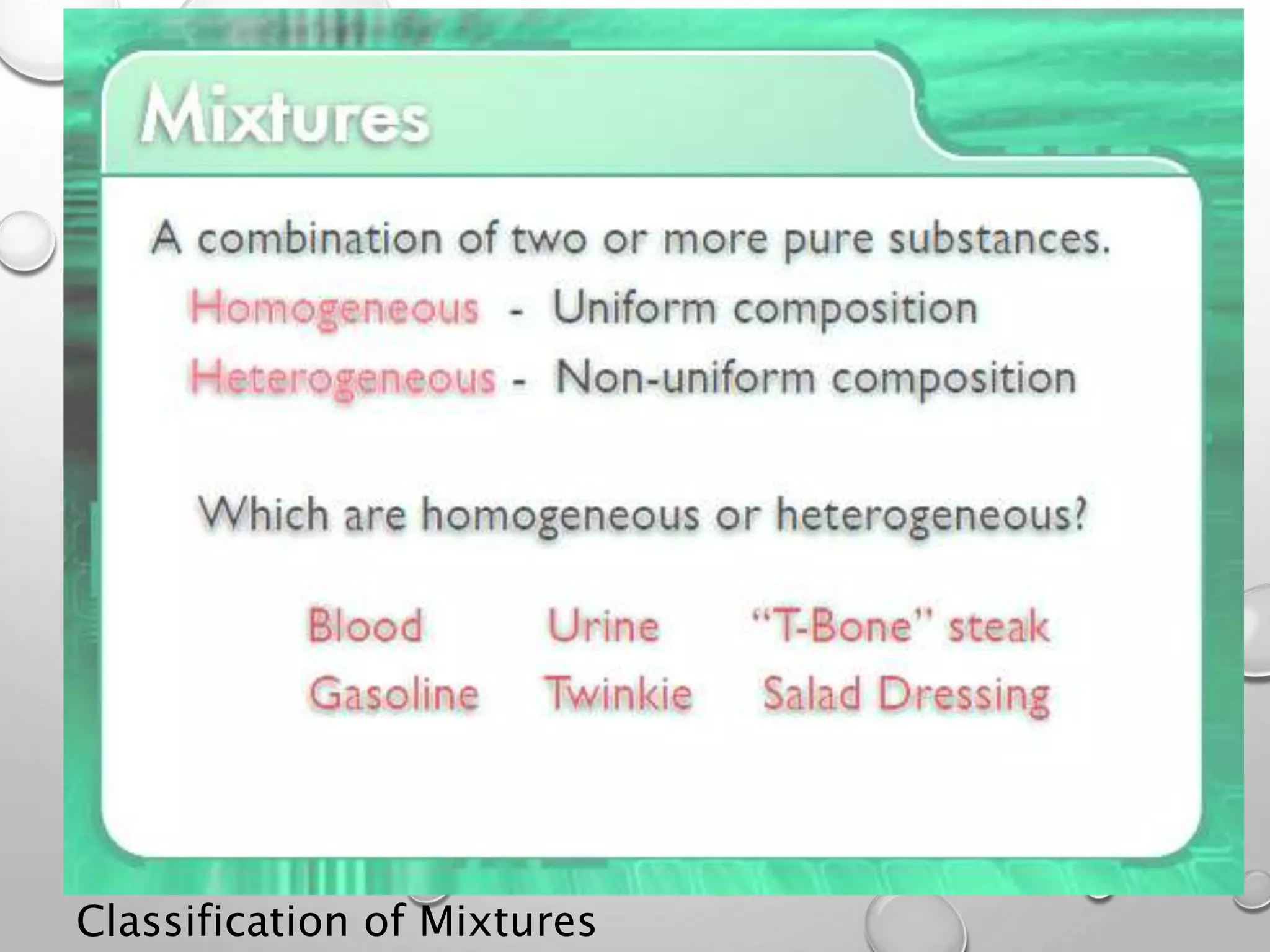
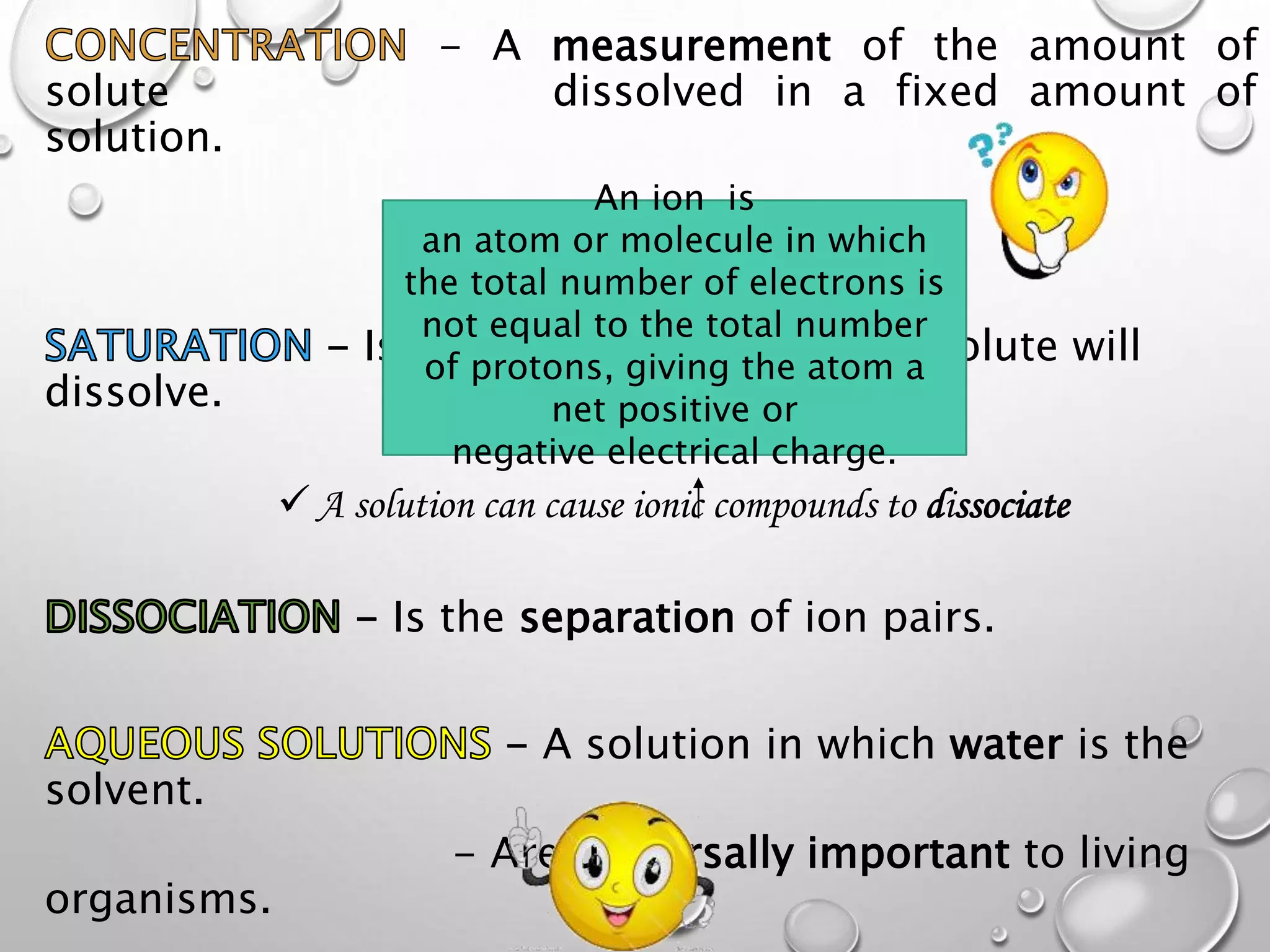
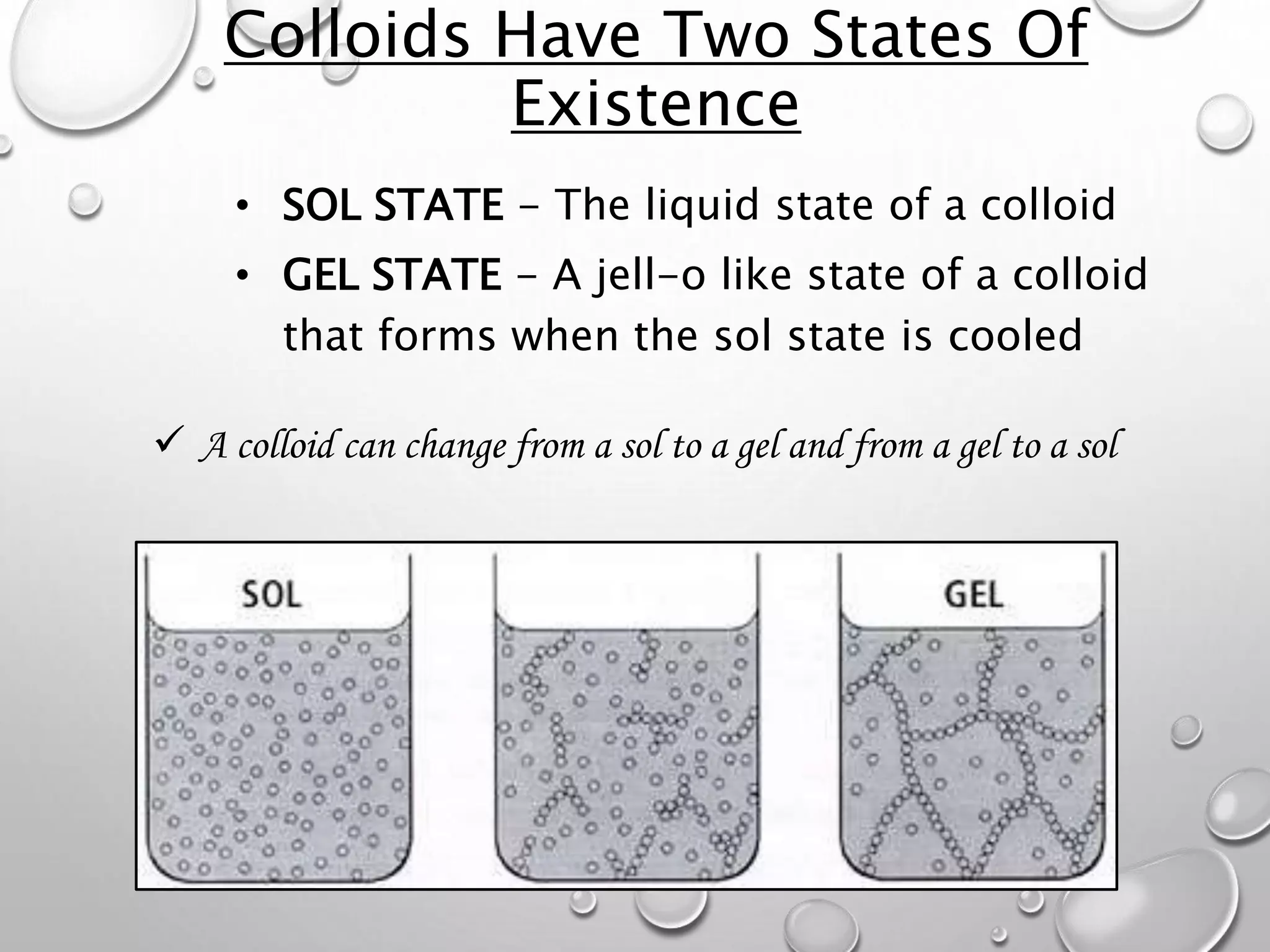
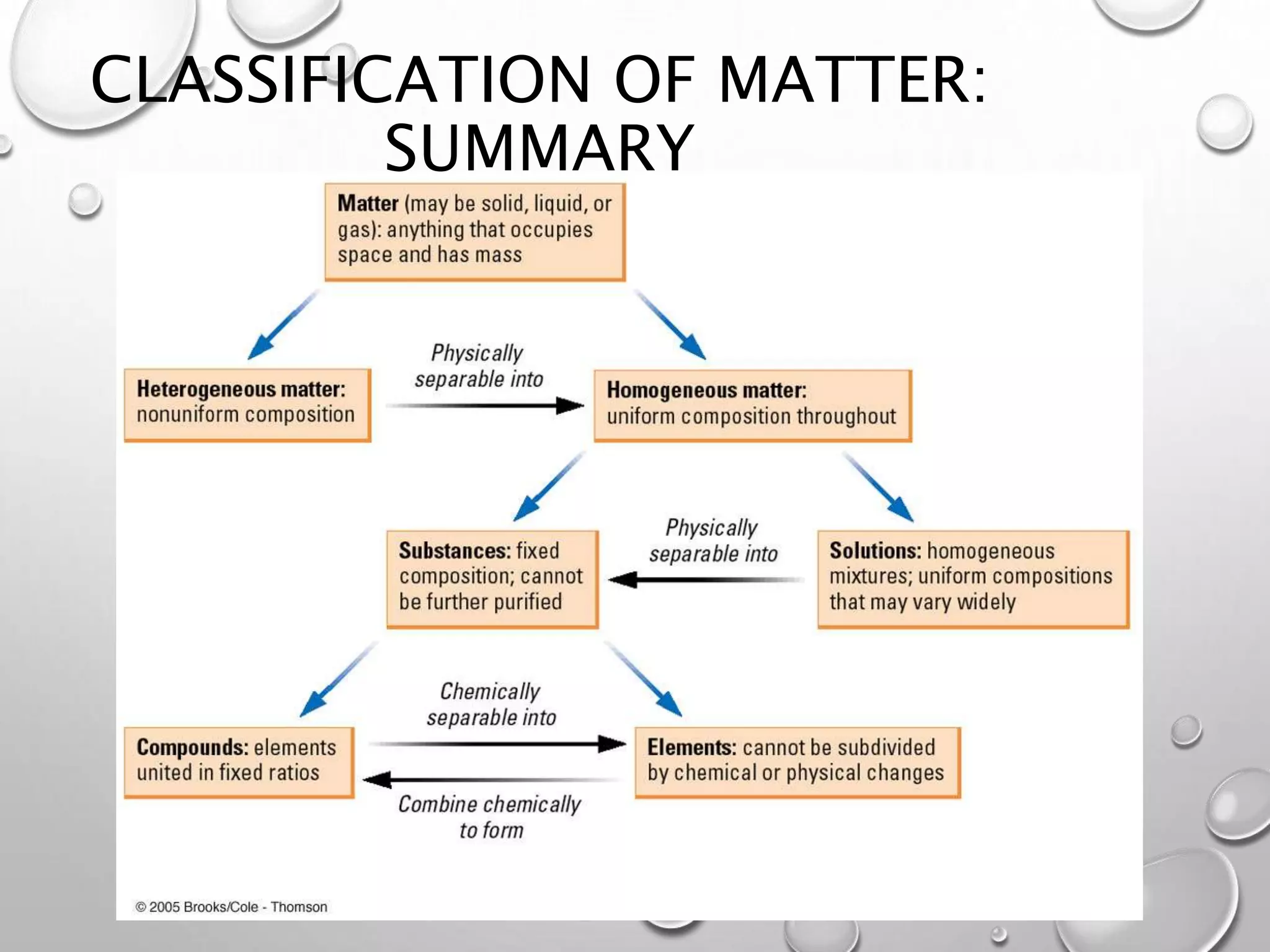
This document discusses the classification of matter. It defines a pure substance as either an element or compound that has a definite and uniform chemical composition and physical properties. Mixtures can be either heterogeneous or homogeneous. A heterogeneous mixture has an uneven texture that is visible, while a homogeneous mixture, or solution, is completely uniform throughout. There are three main types of mixtures: solutions, suspensions, and colloids. A solution is a mixture where one or more substances are uniformly distributed in another substance. A suspension is a mixture where particles are spread through but settle over time. A colloid is between a solution and suspension, with particles that do not settle over time.