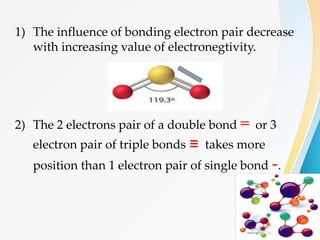
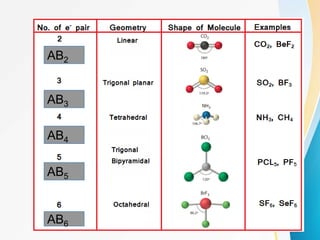
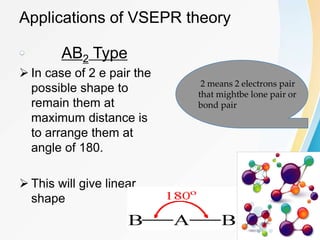
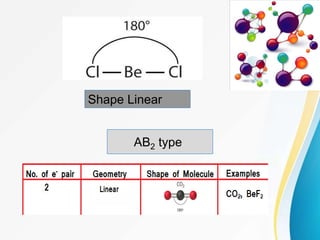
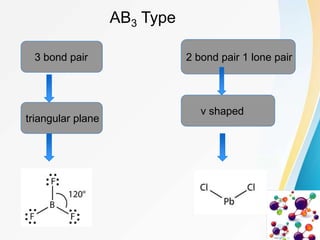
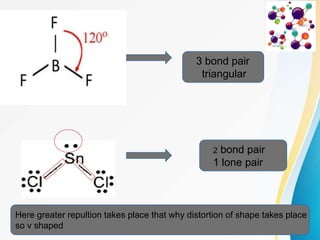
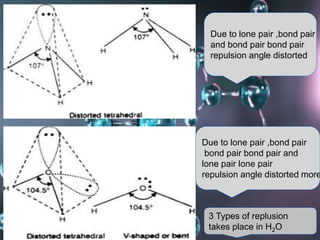
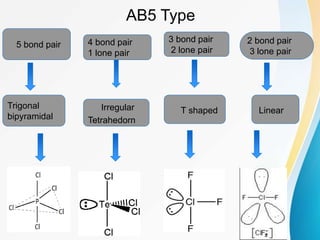
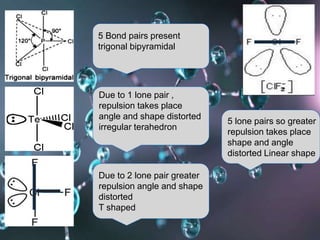
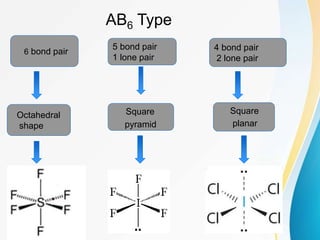
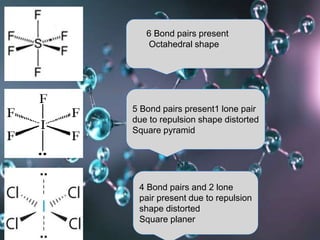
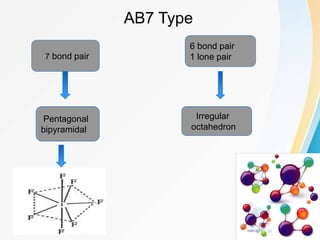
VSEPR (Valence Shell Electron Pair Repulsion) theory predicts molecular geometry based on the repulsion of electron pairs in the valence shell of an atom. It states that electron pairs will adopt a geometry that minimizes repulsion by maximizing distance between electron pairs. The number and type of electron pairs (lone pairs or bond pairs) determines the molecular shape. Lone pairs occupy more space than bond pairs due to greater repulsion from the single nucleus. Molecular geometry can be distorted from ideal shapes by the presence of lone pairs.