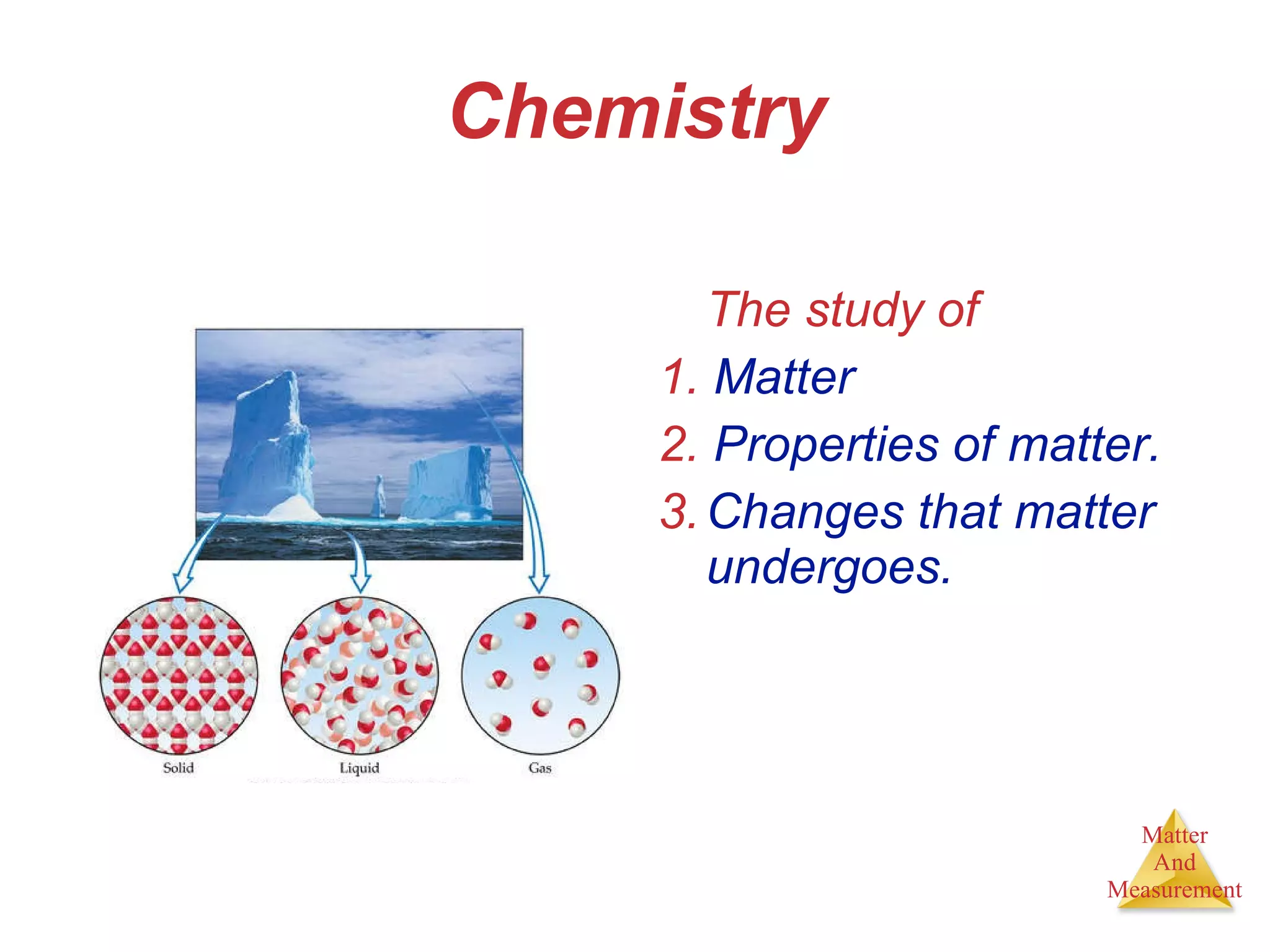
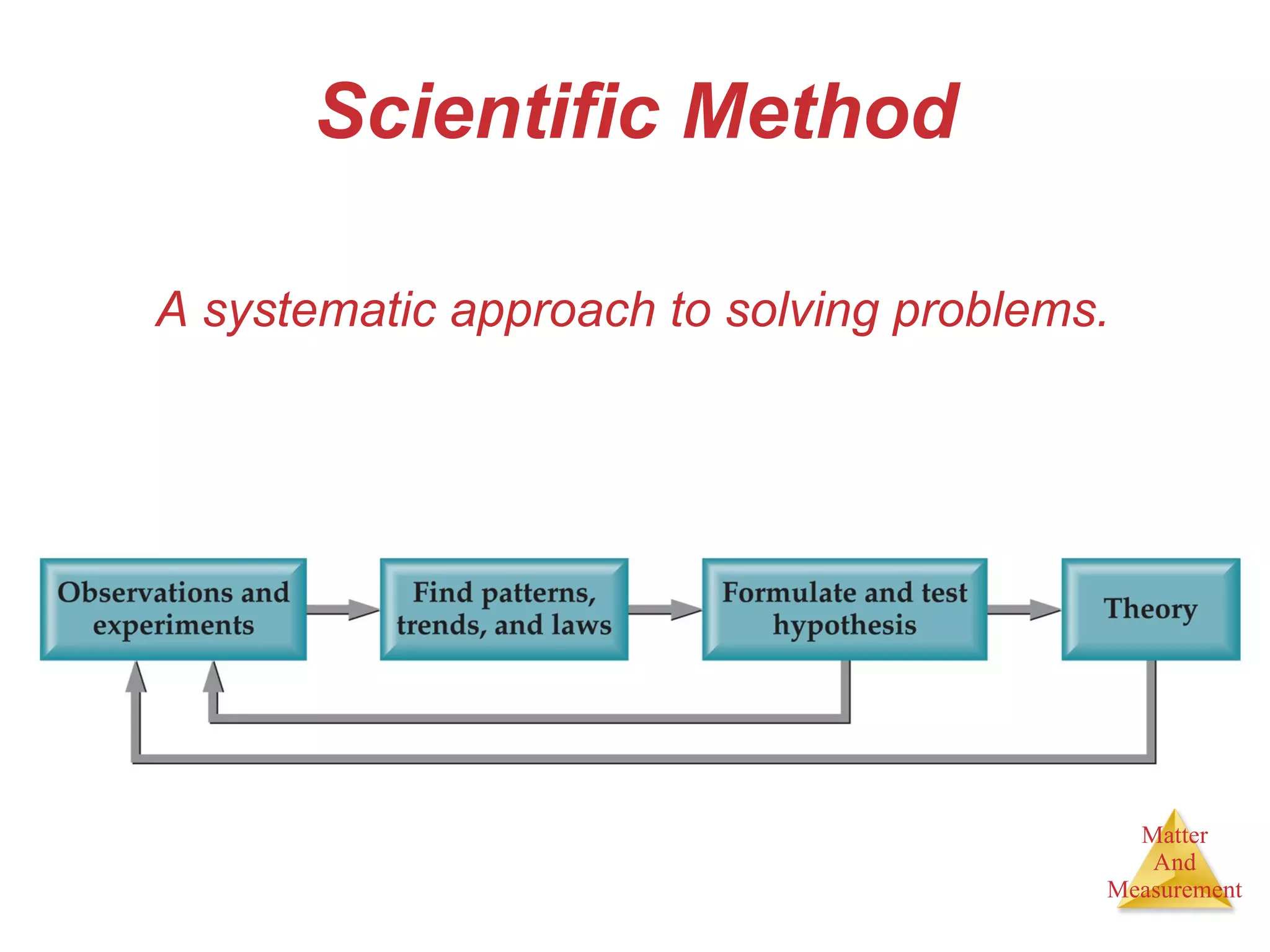
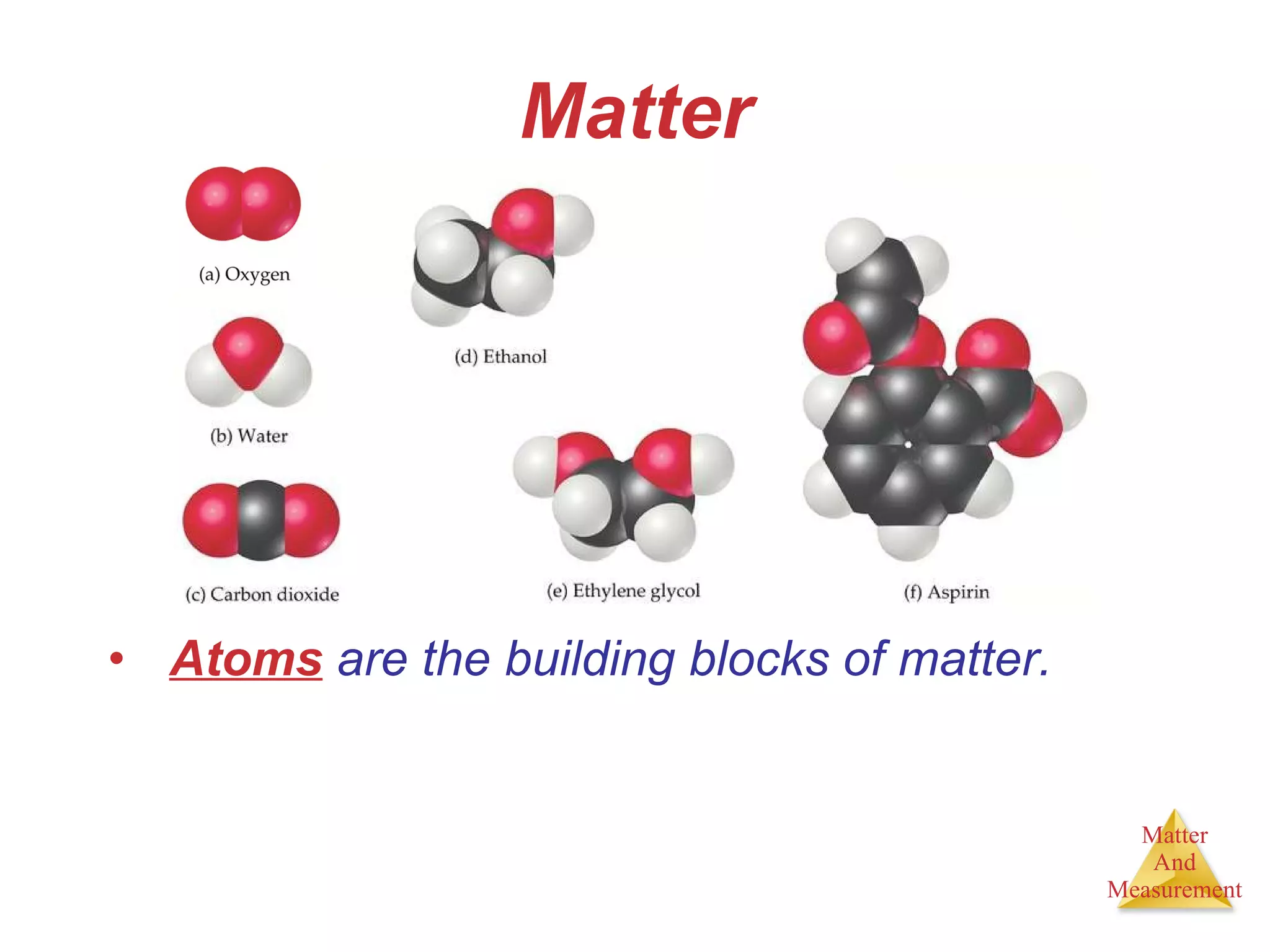
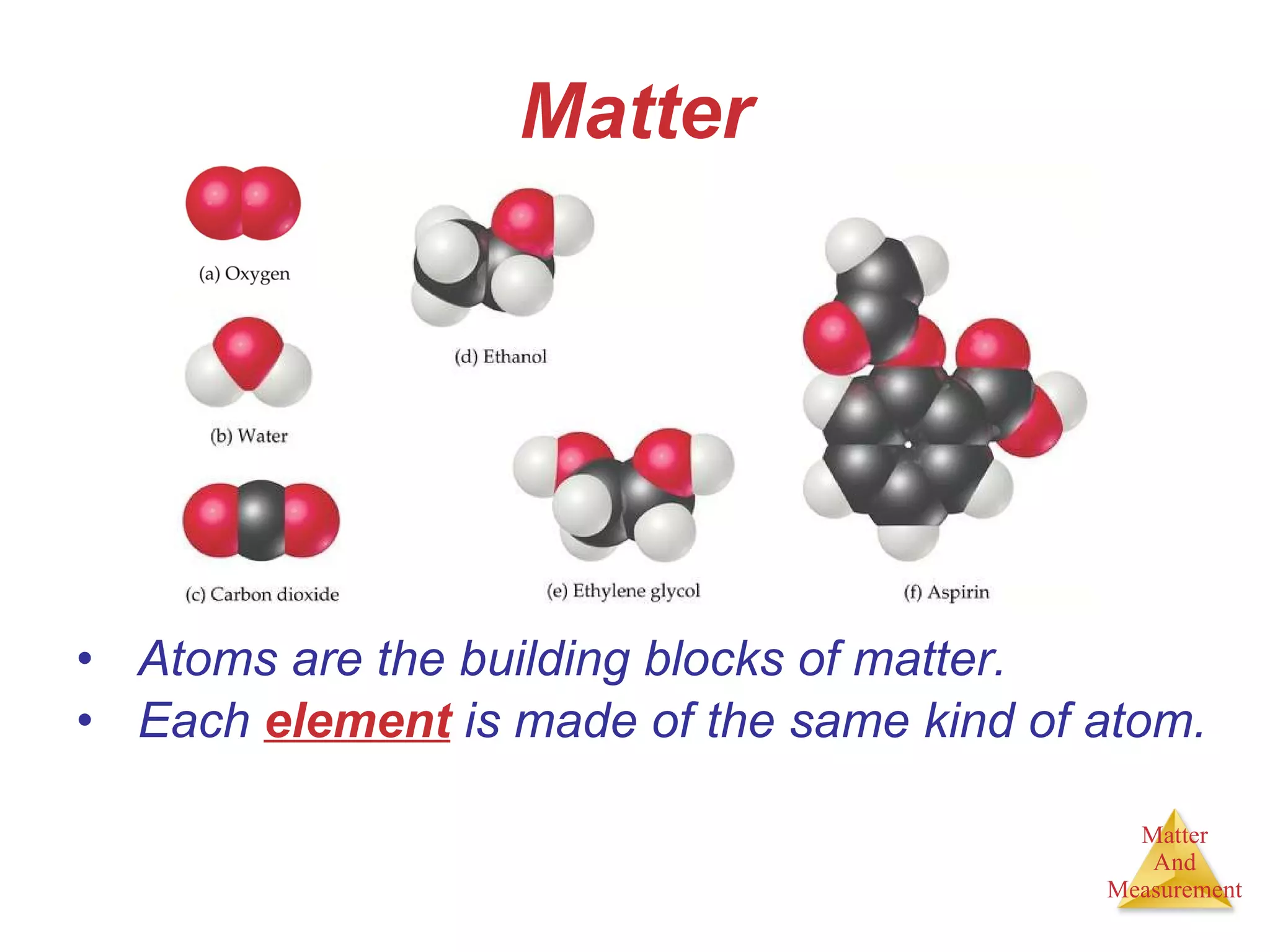
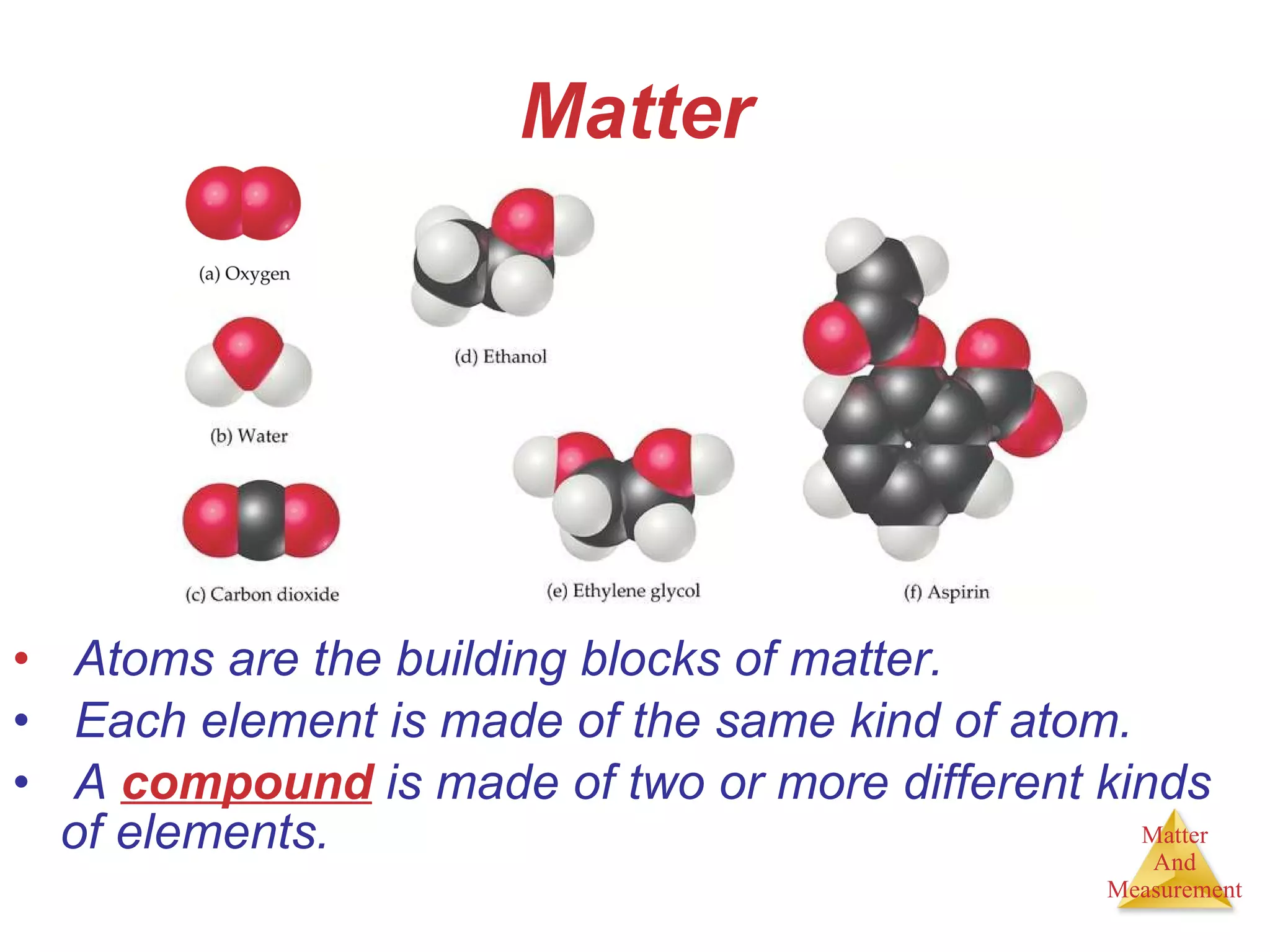
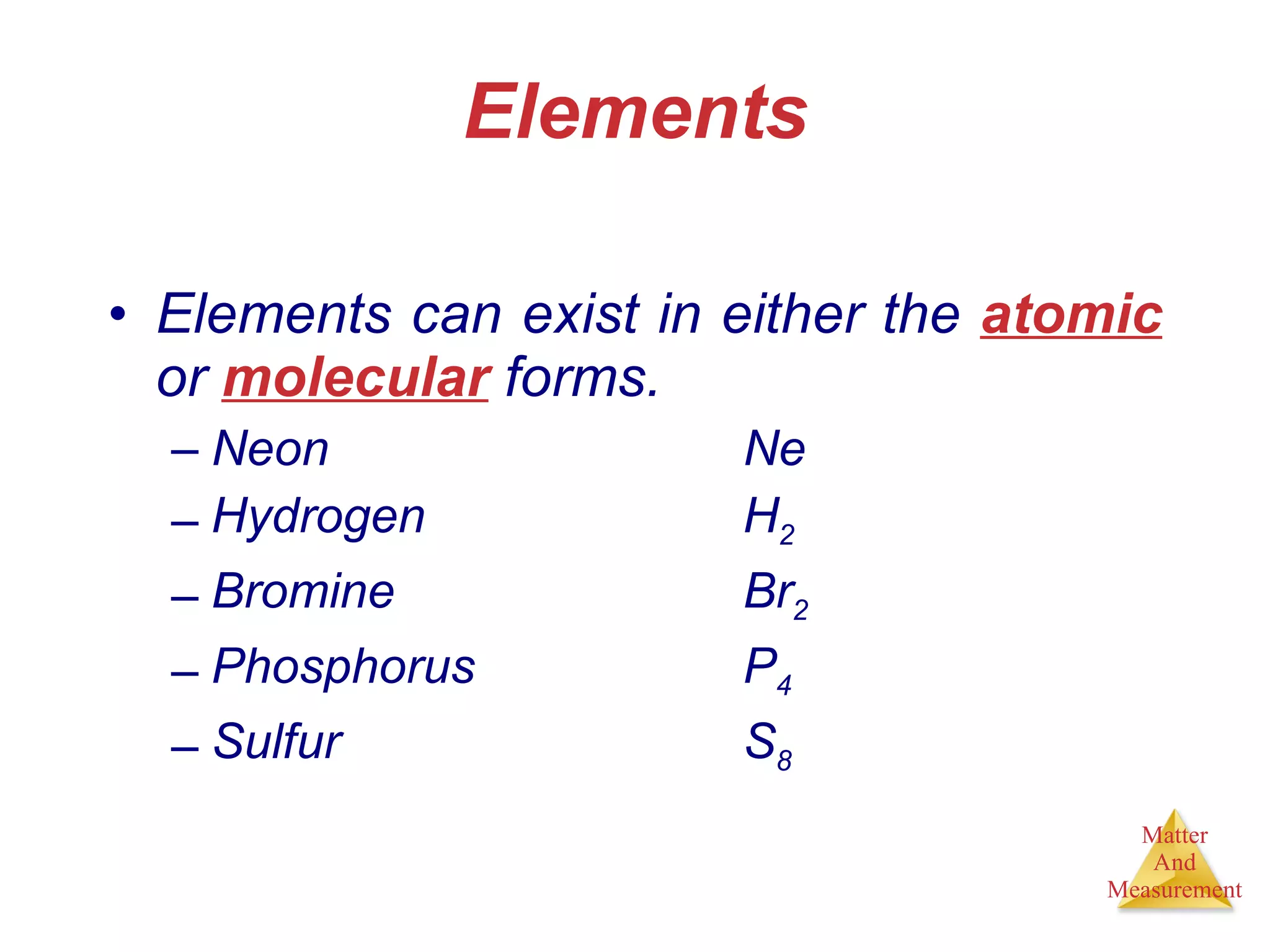
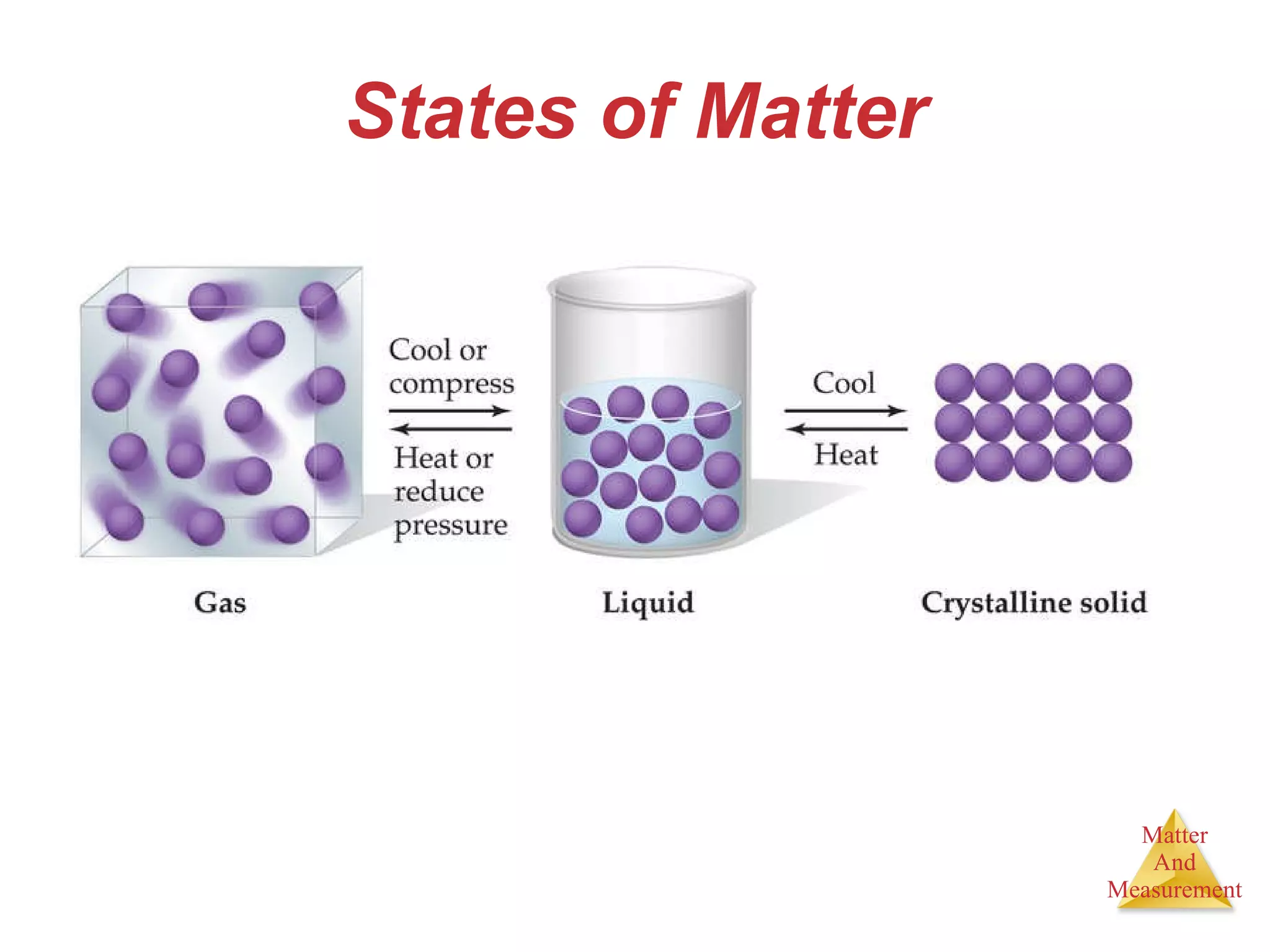
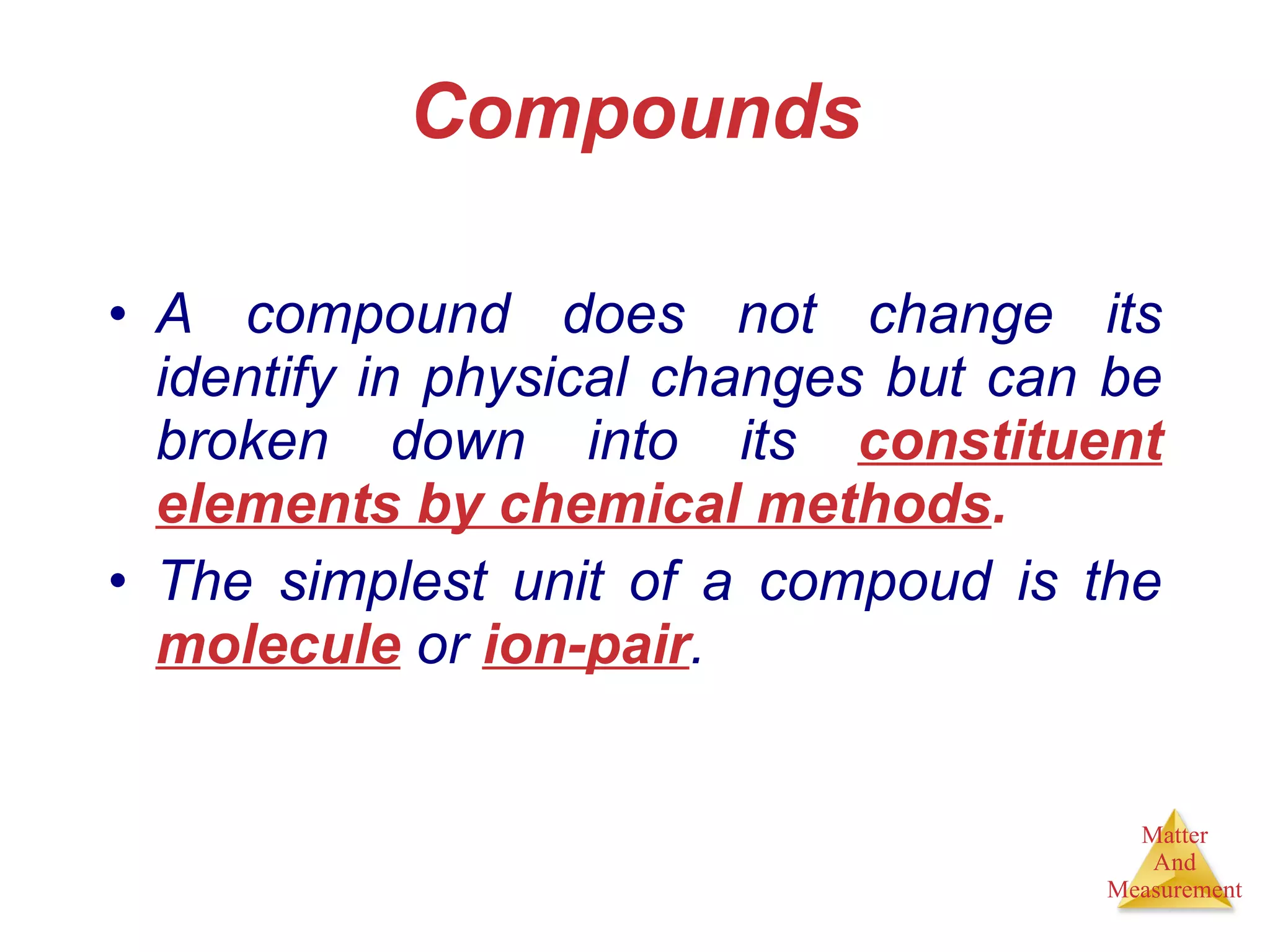
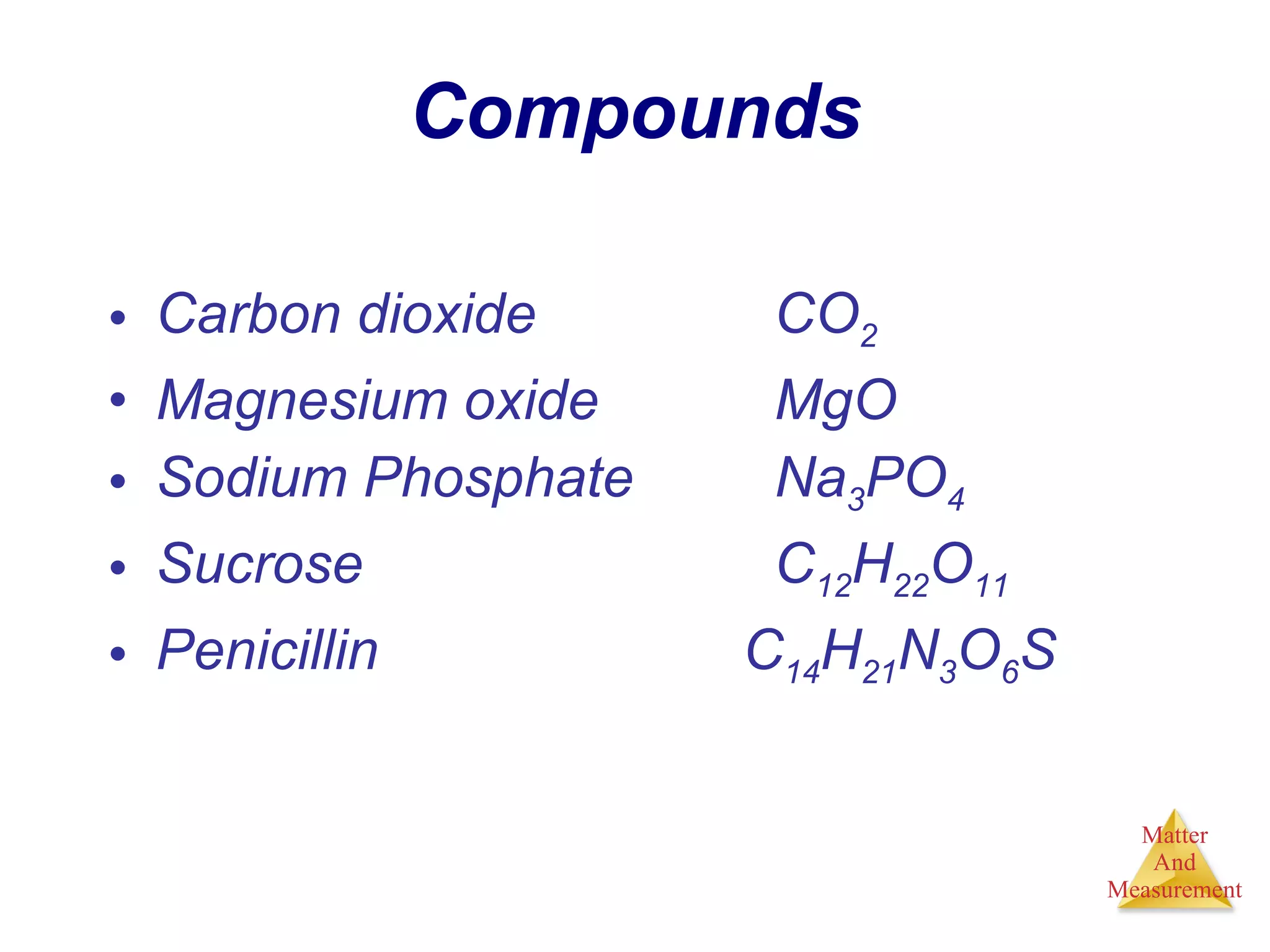
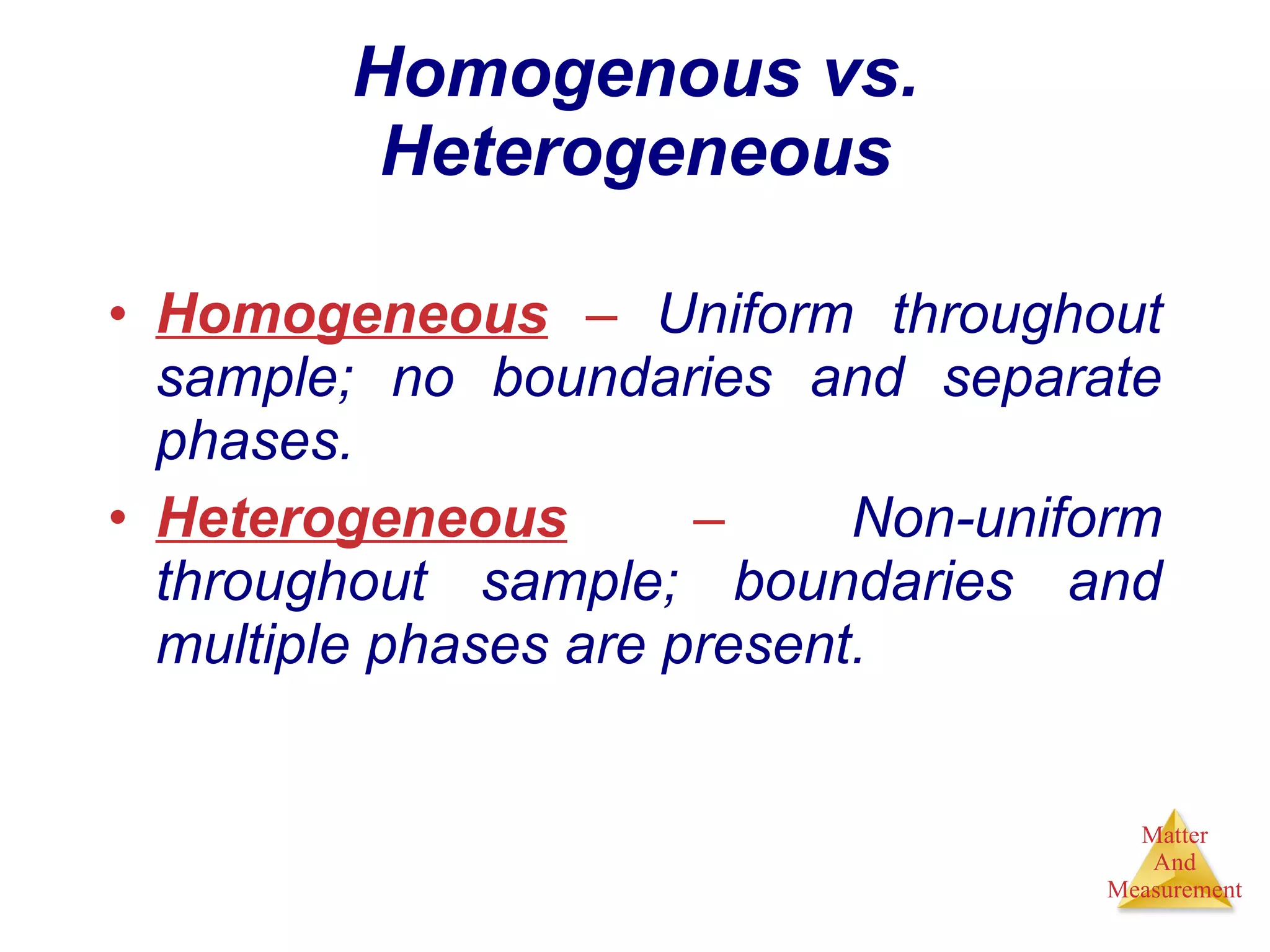
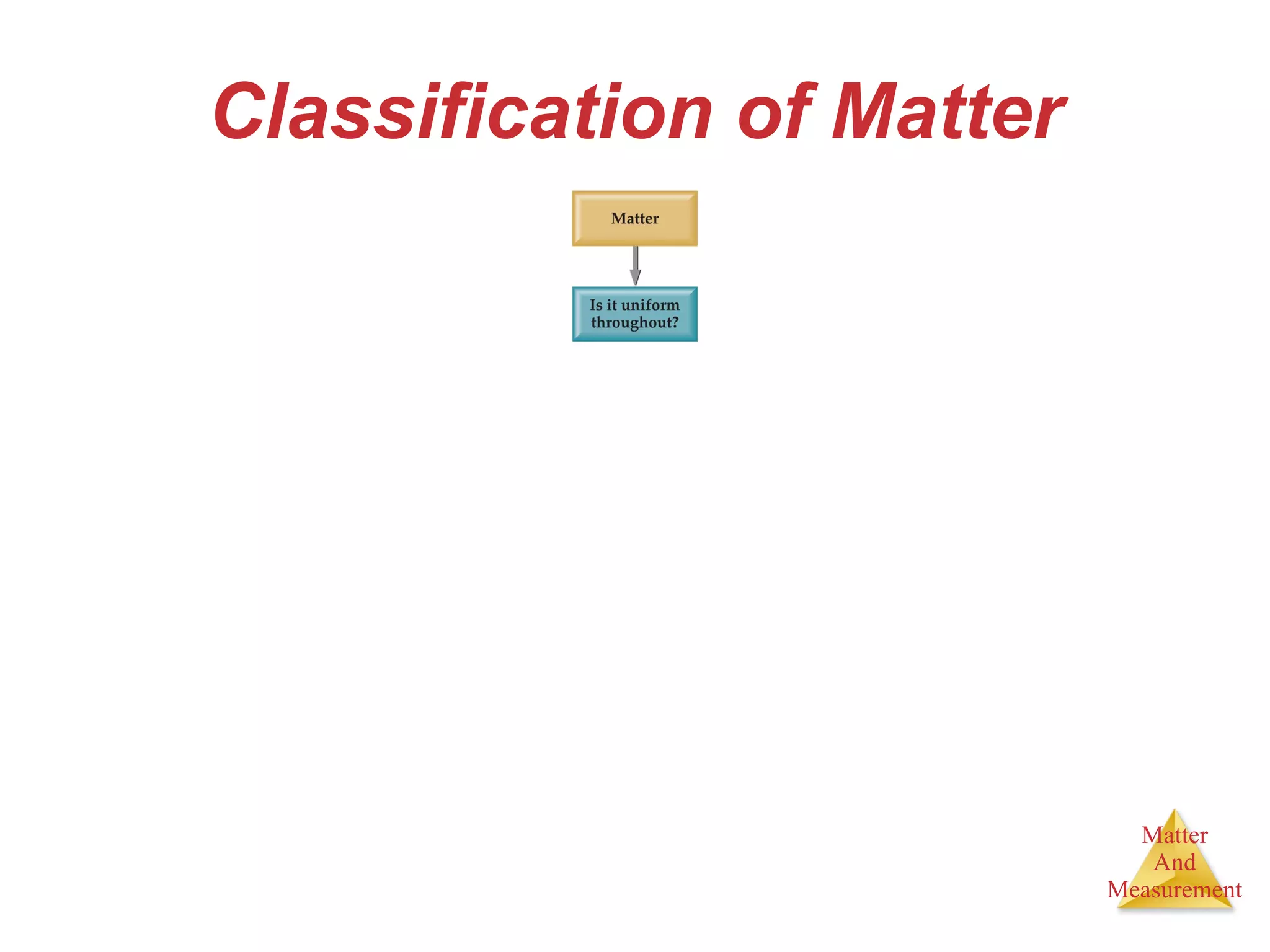
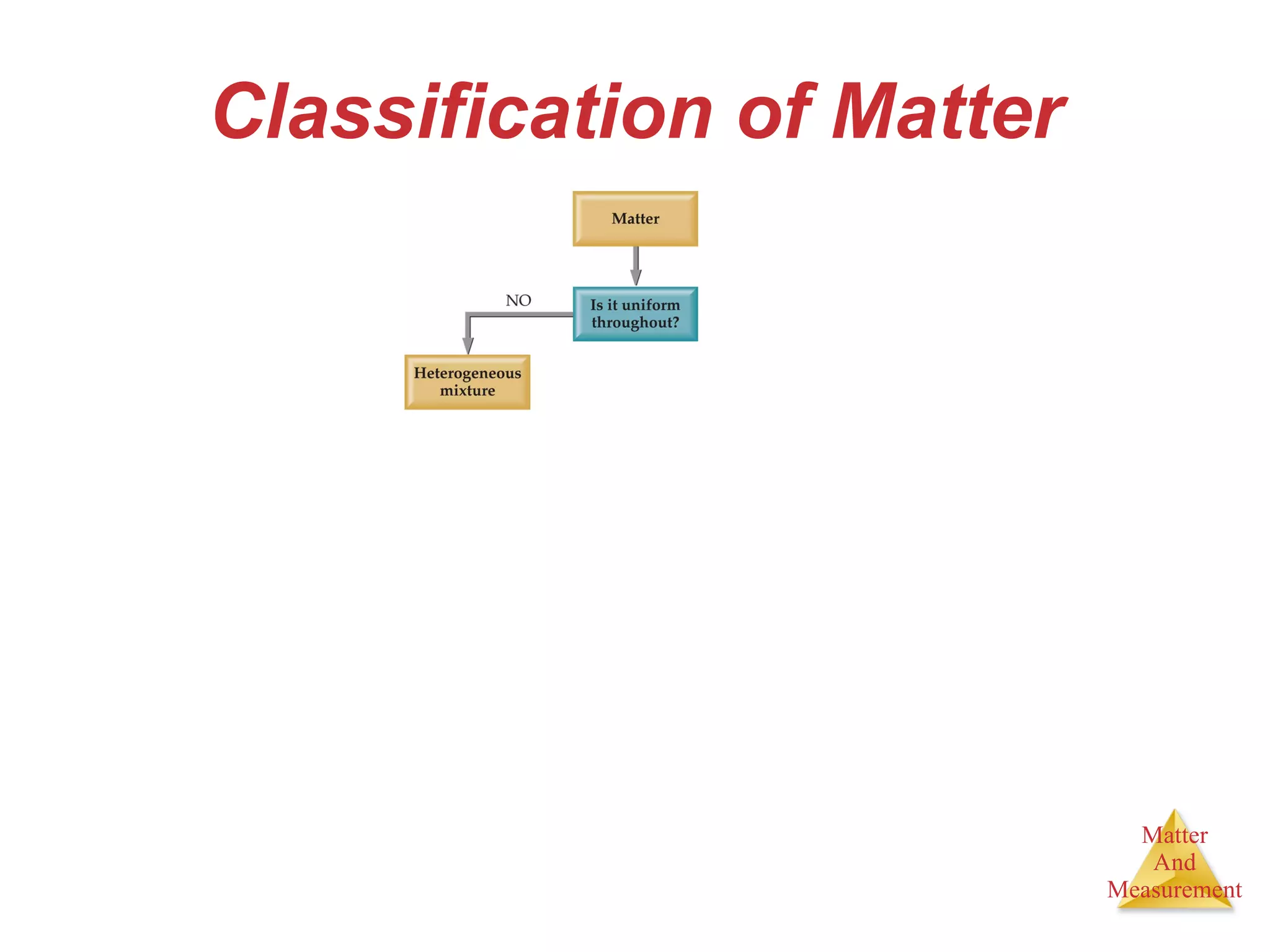
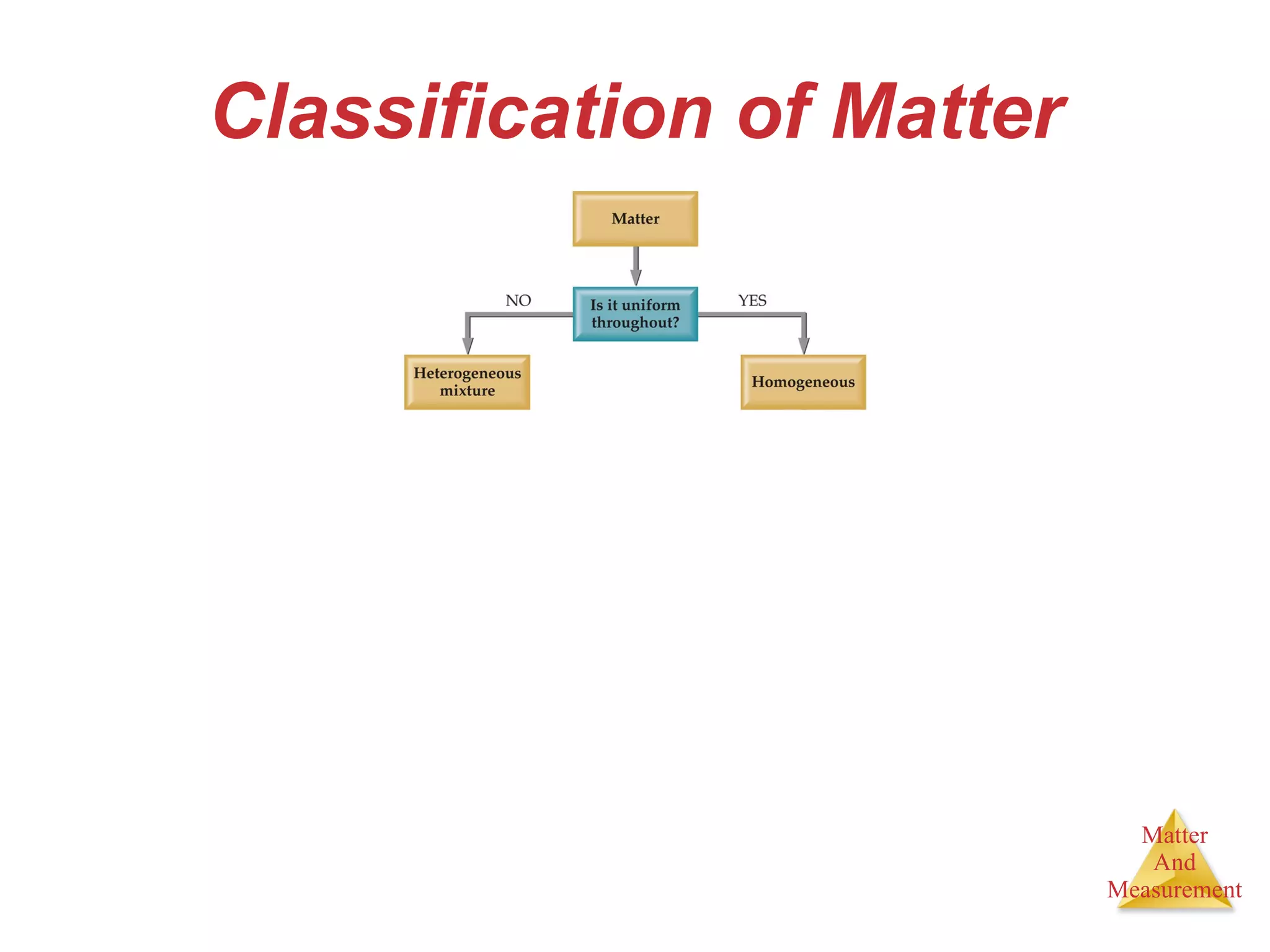
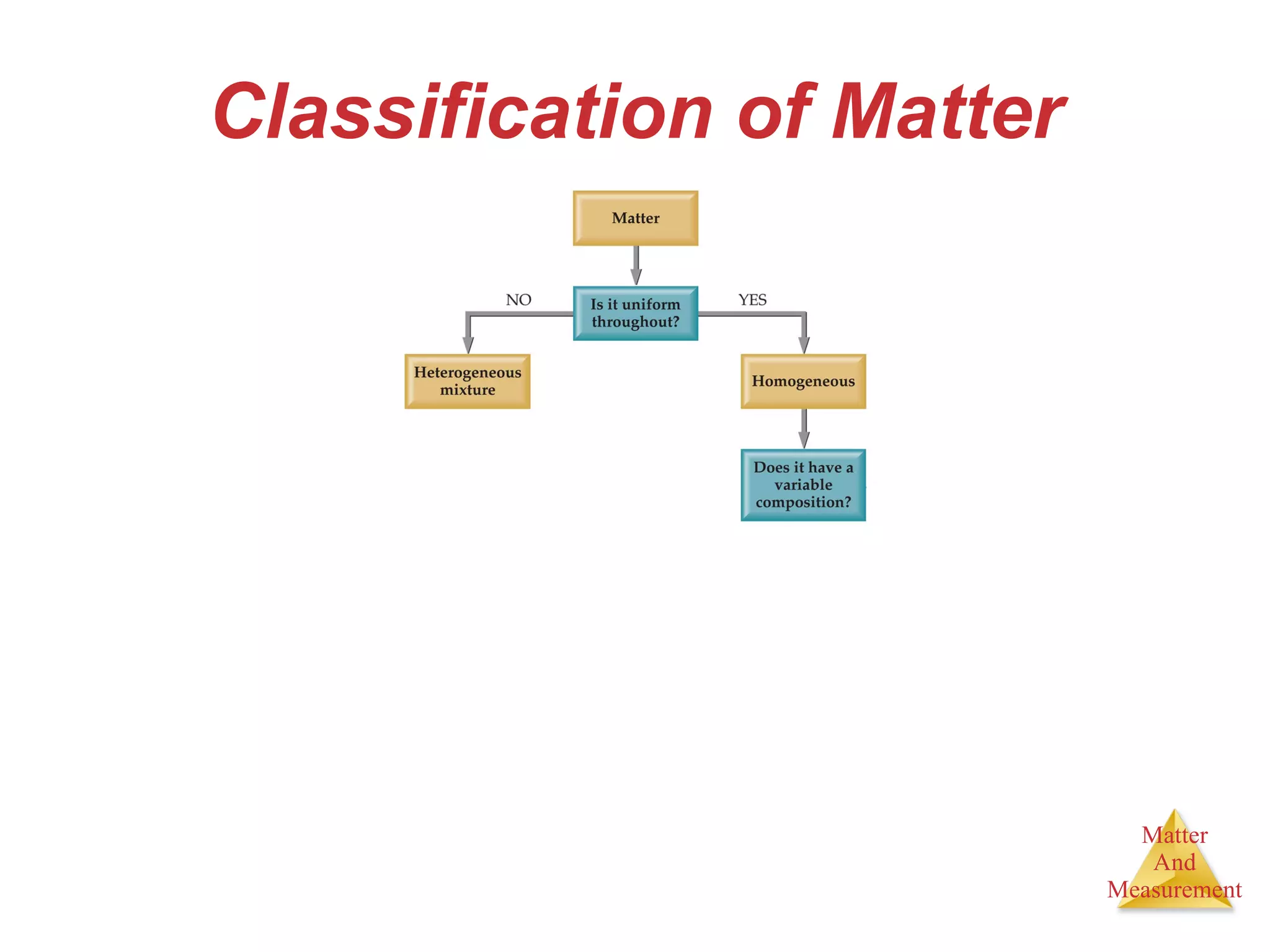
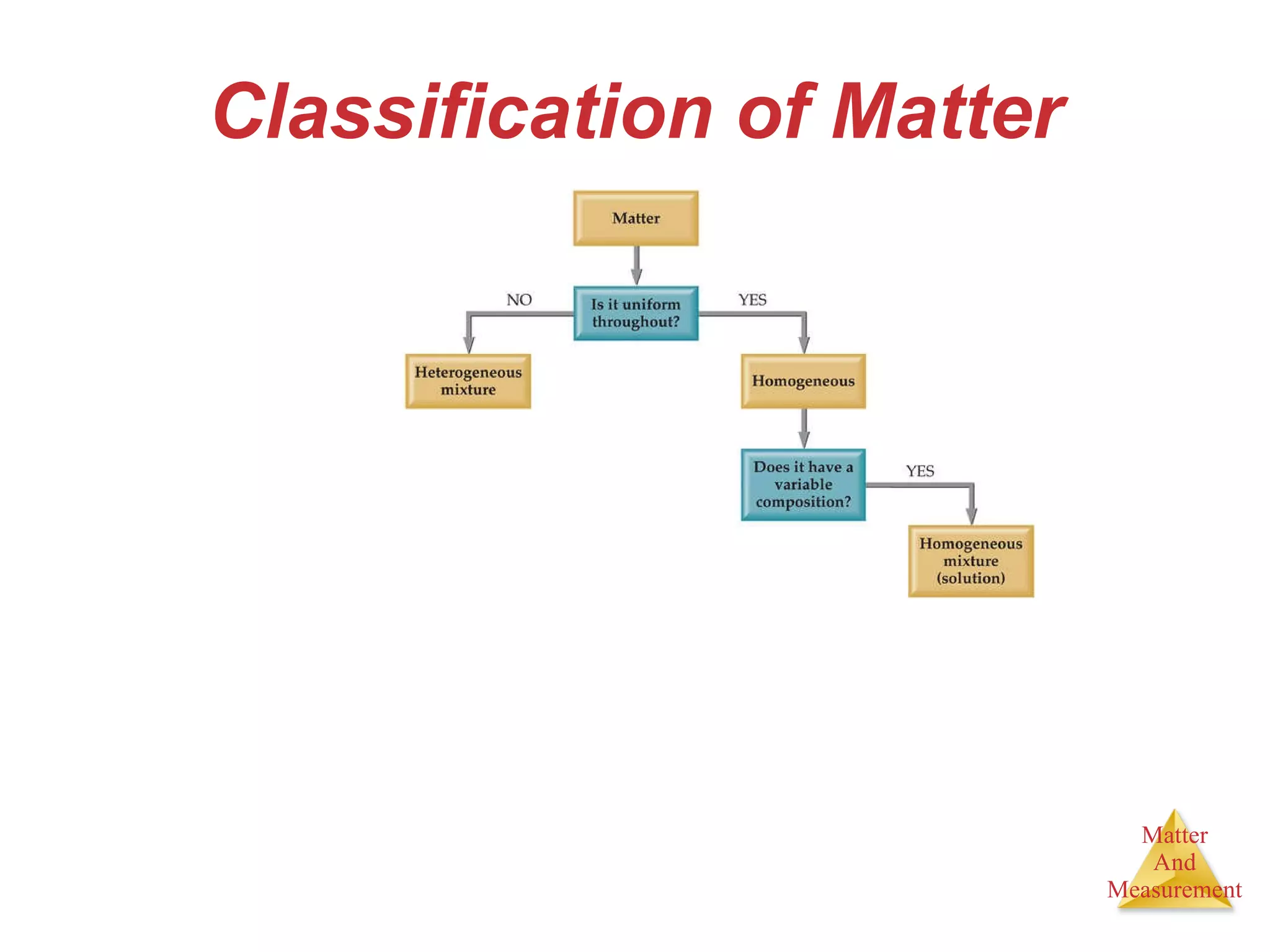
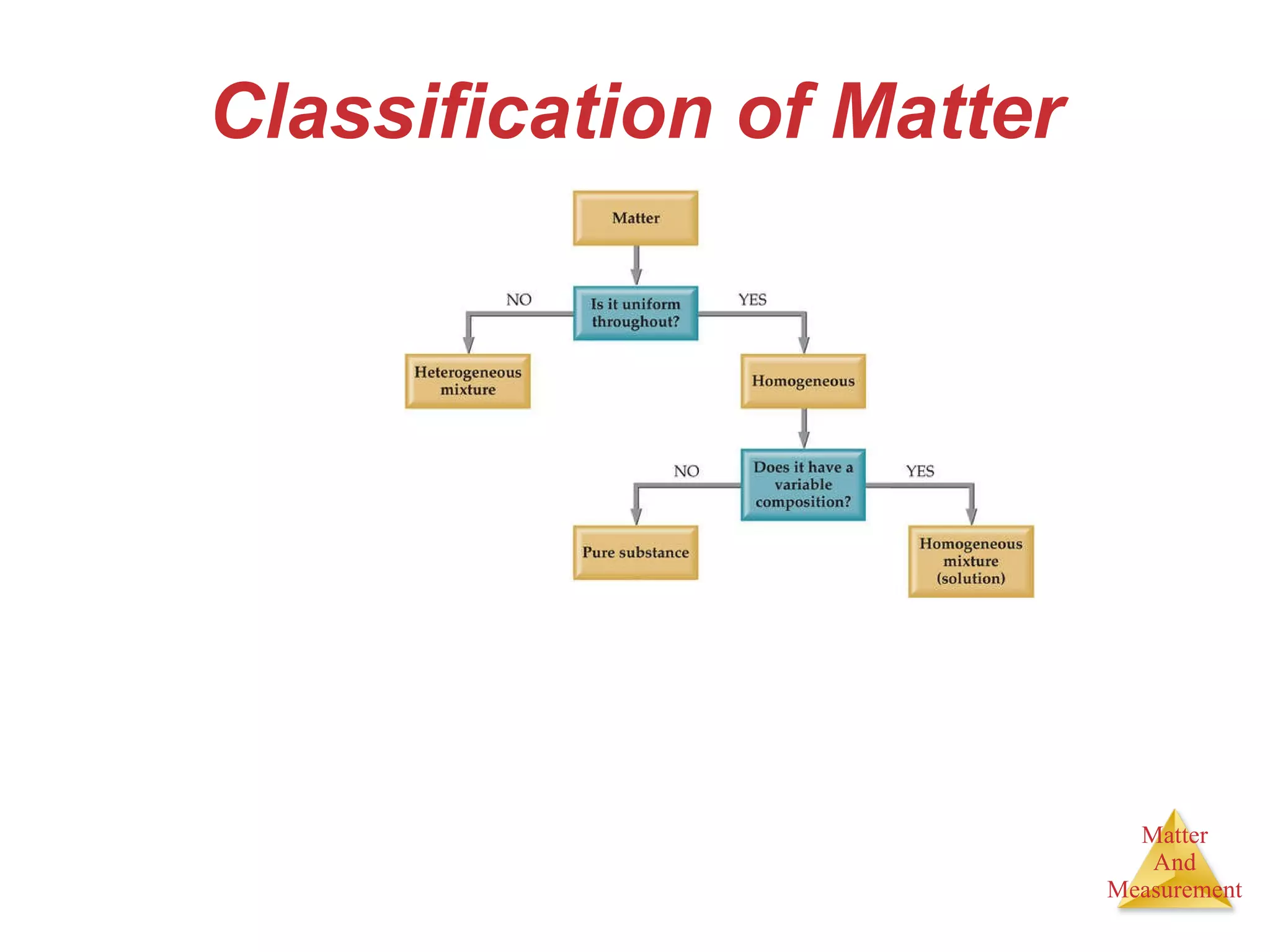
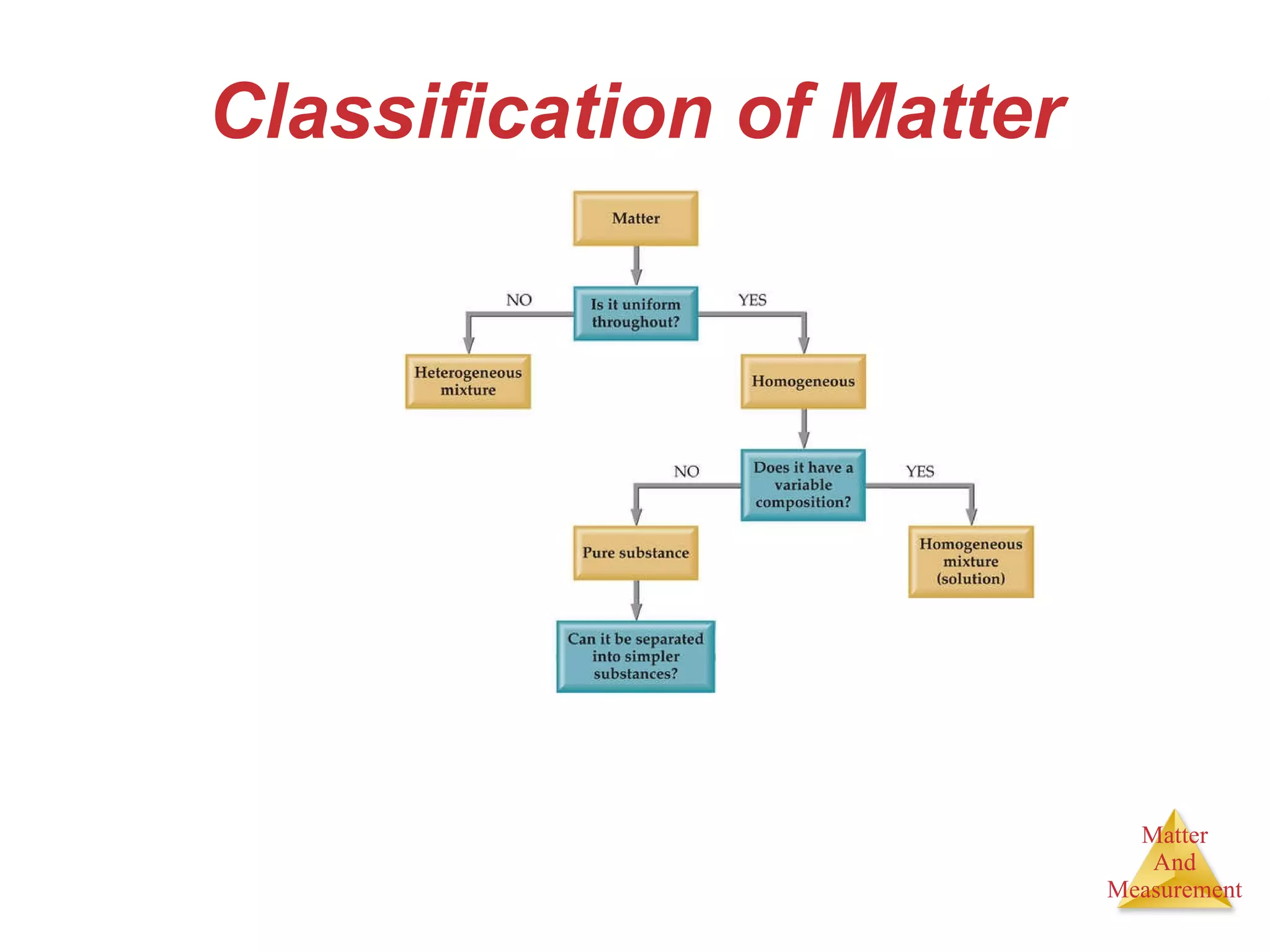
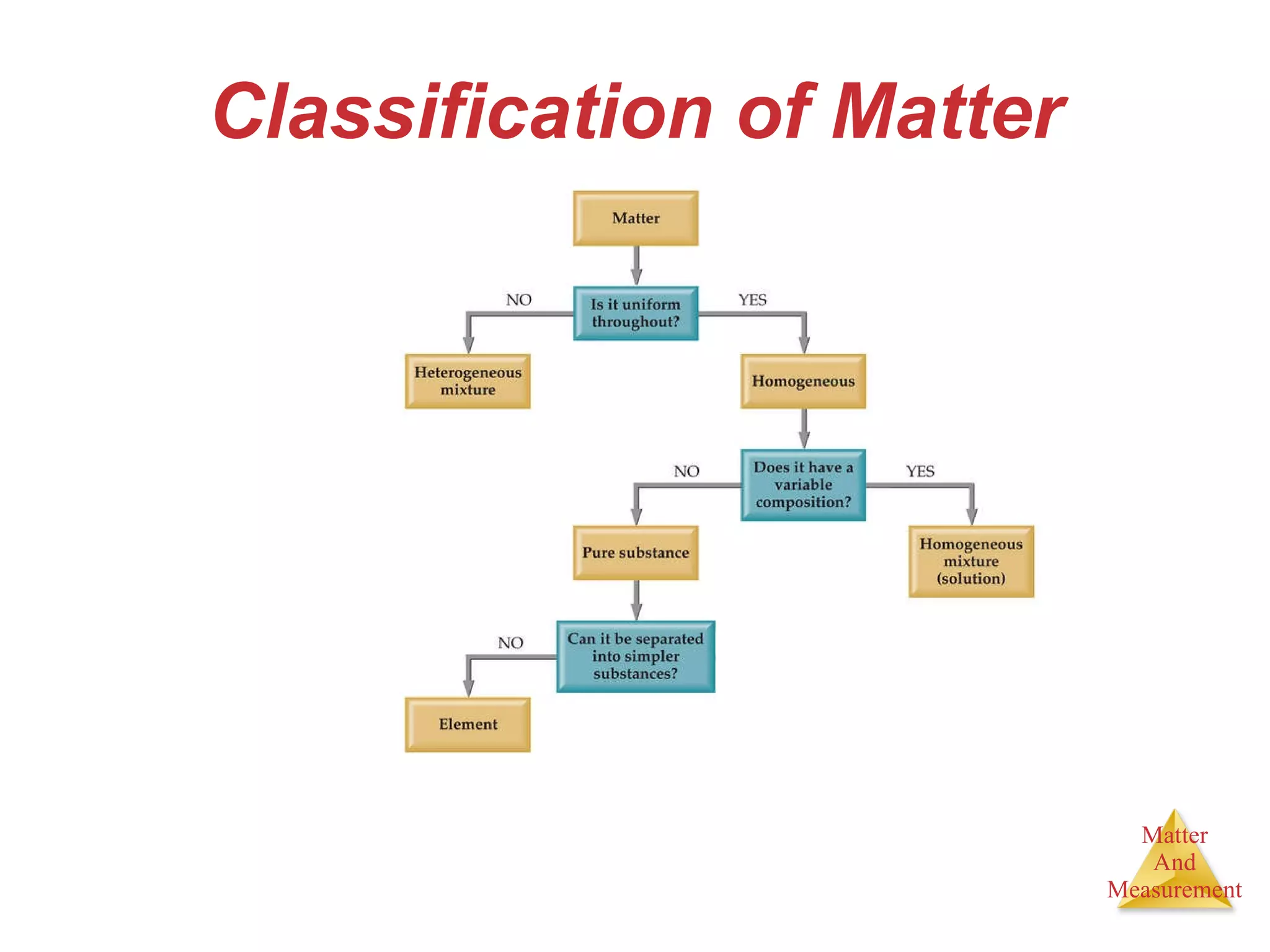
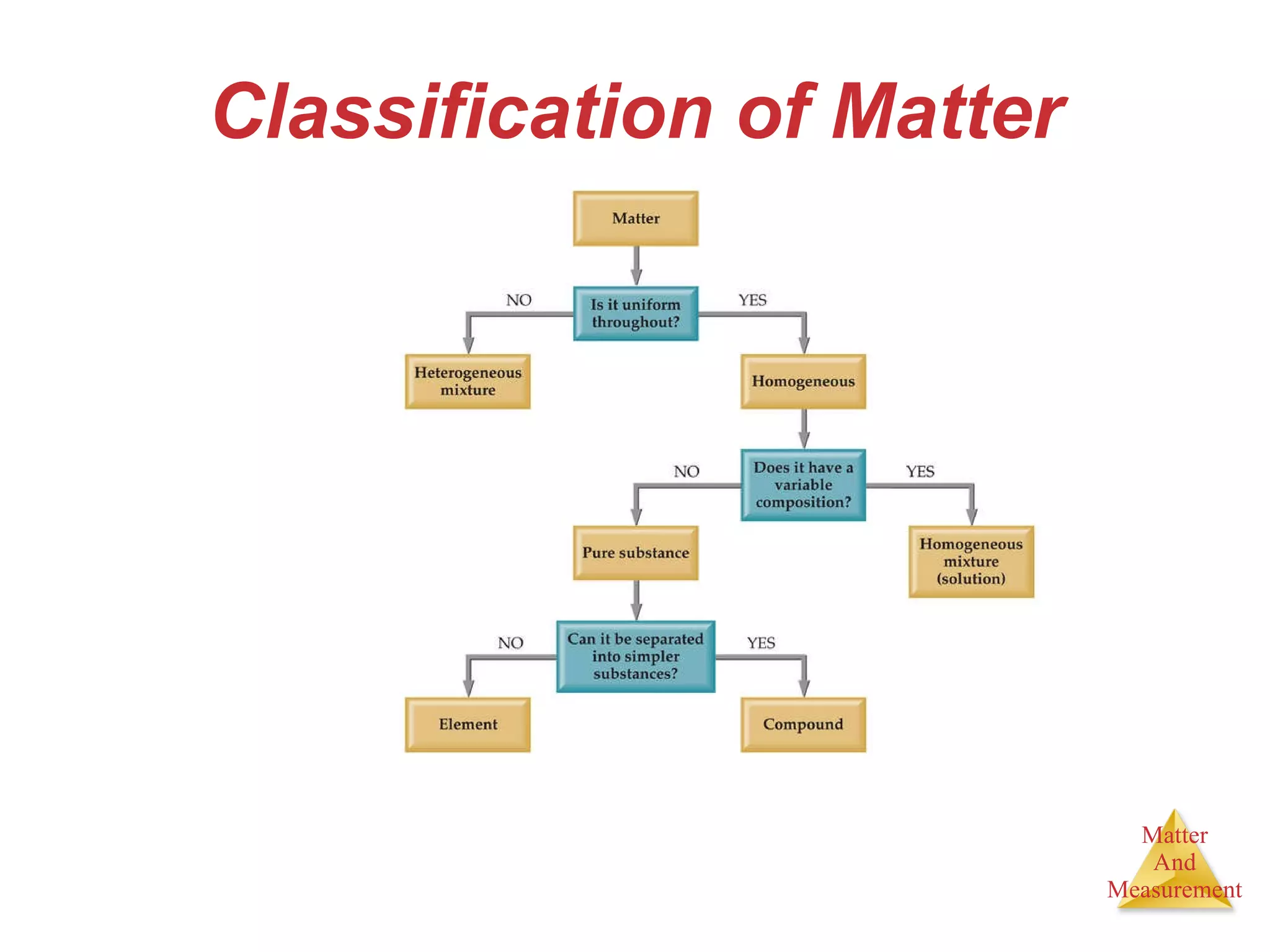
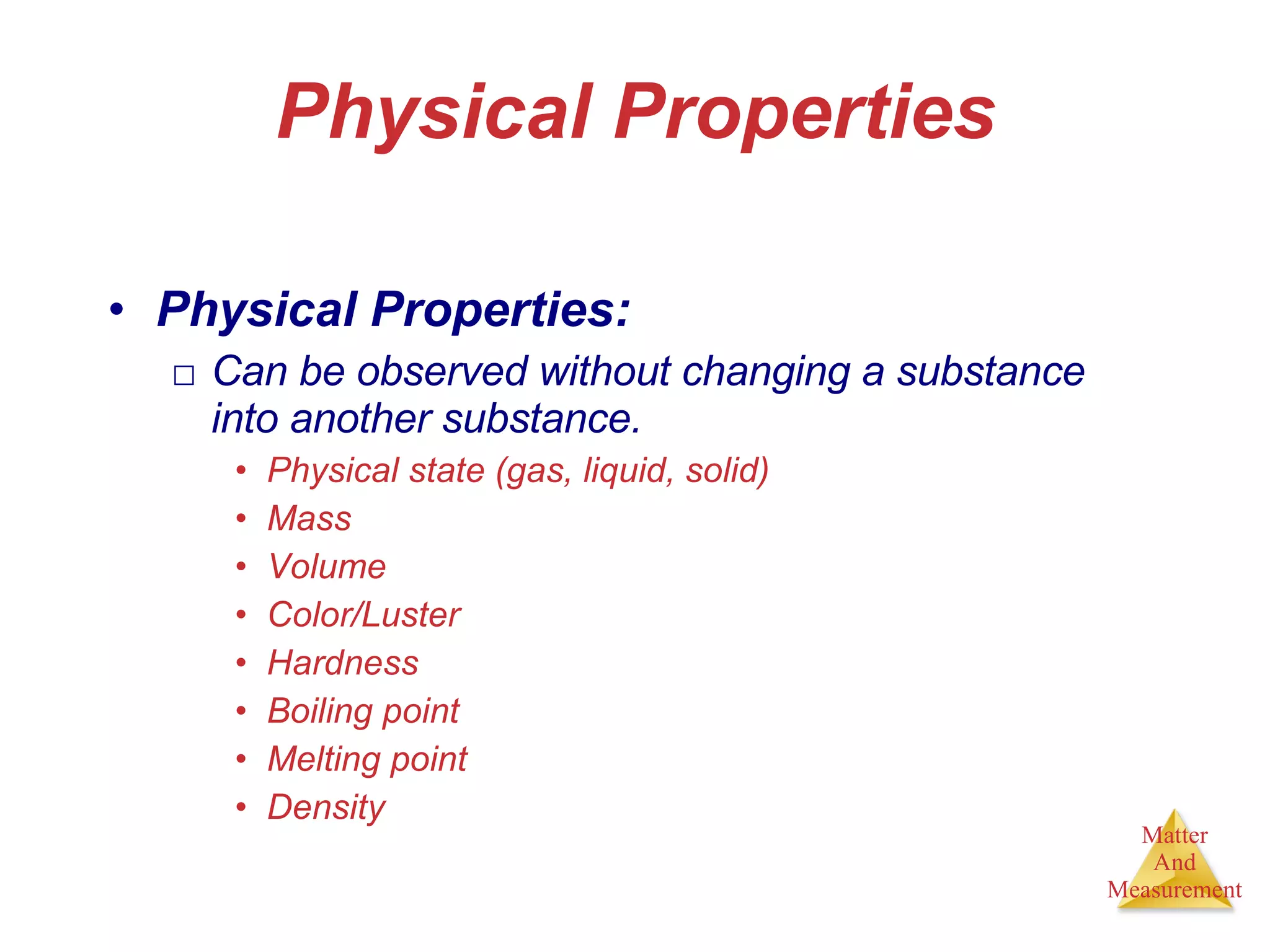
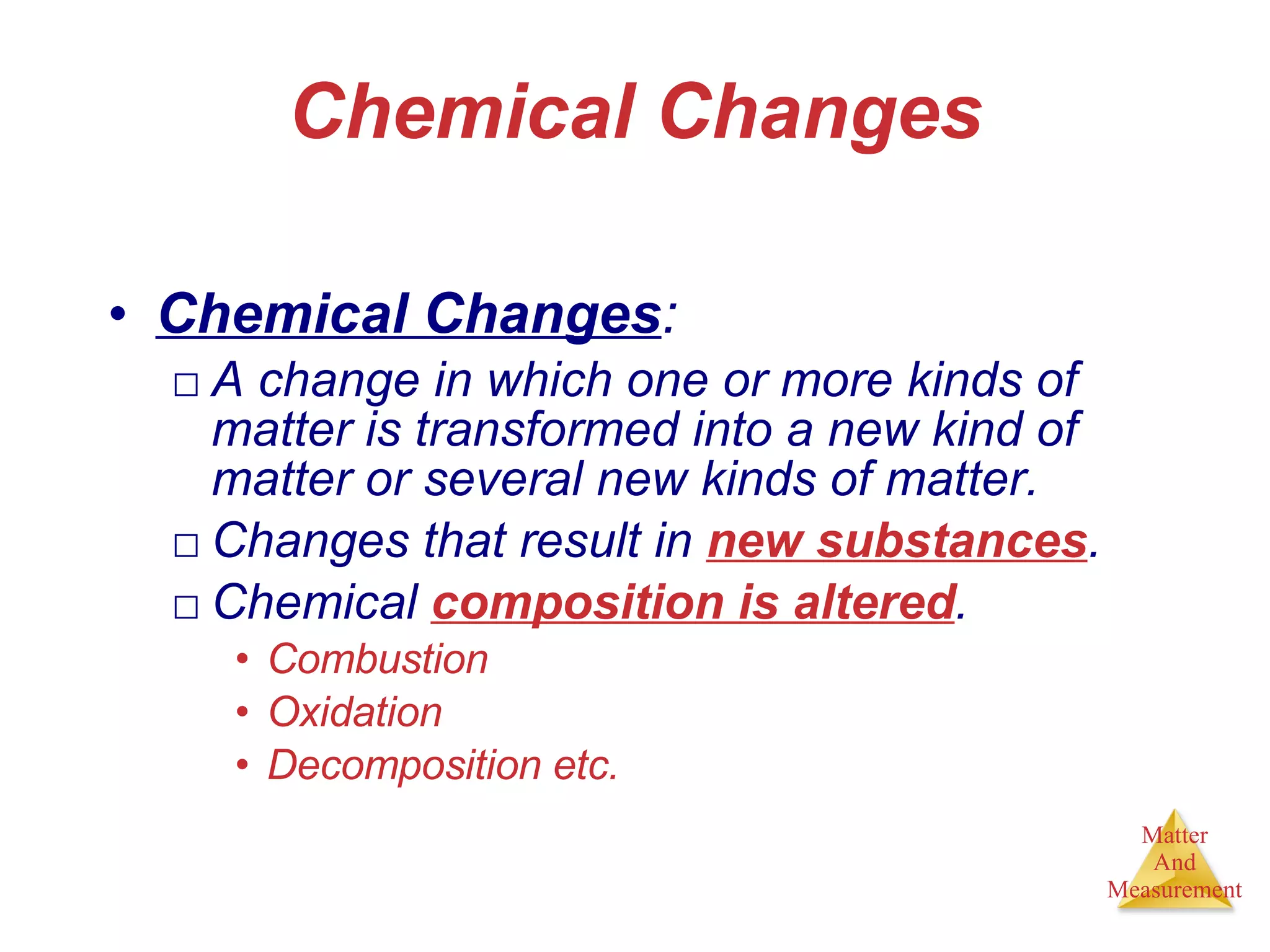
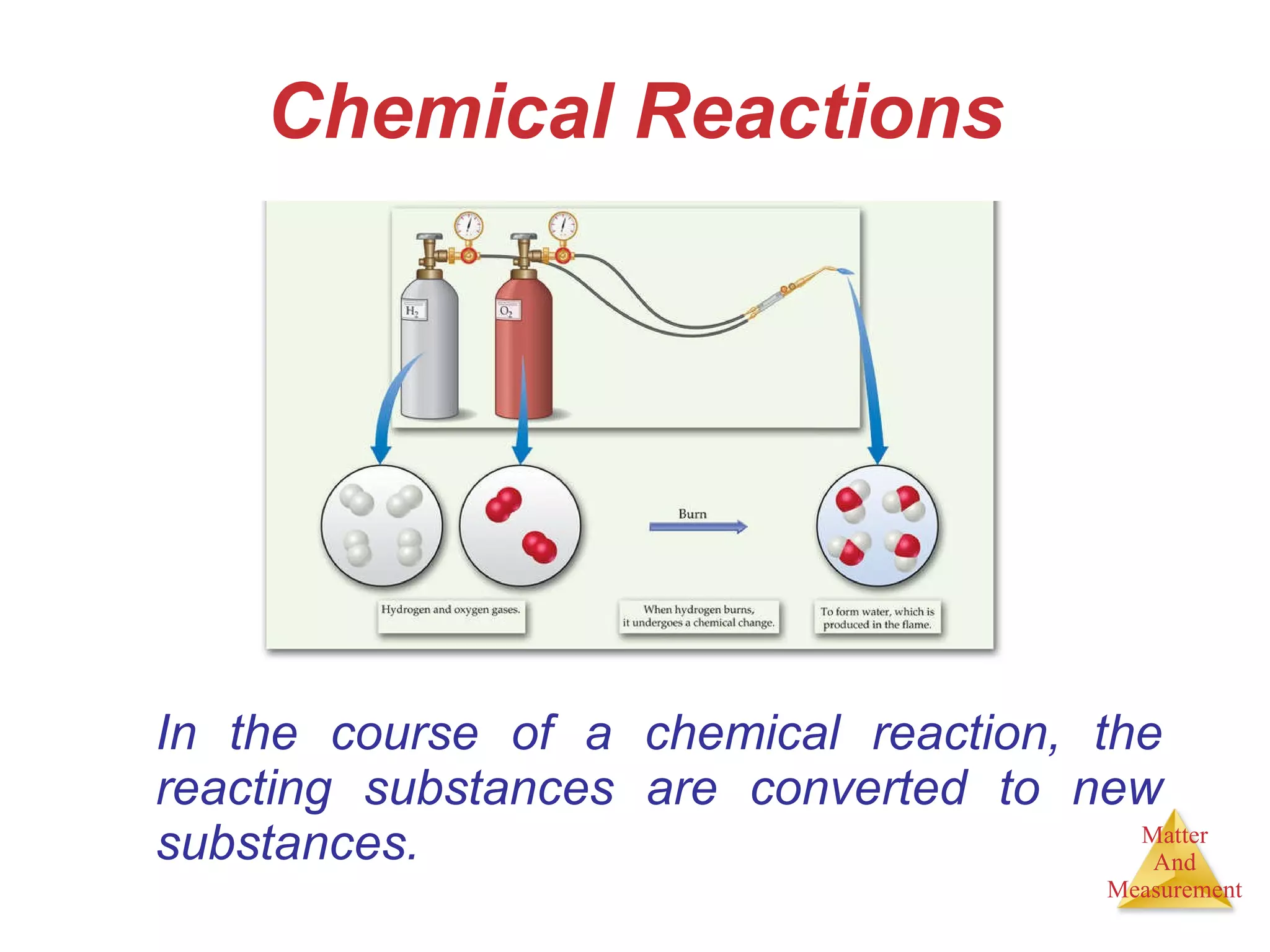
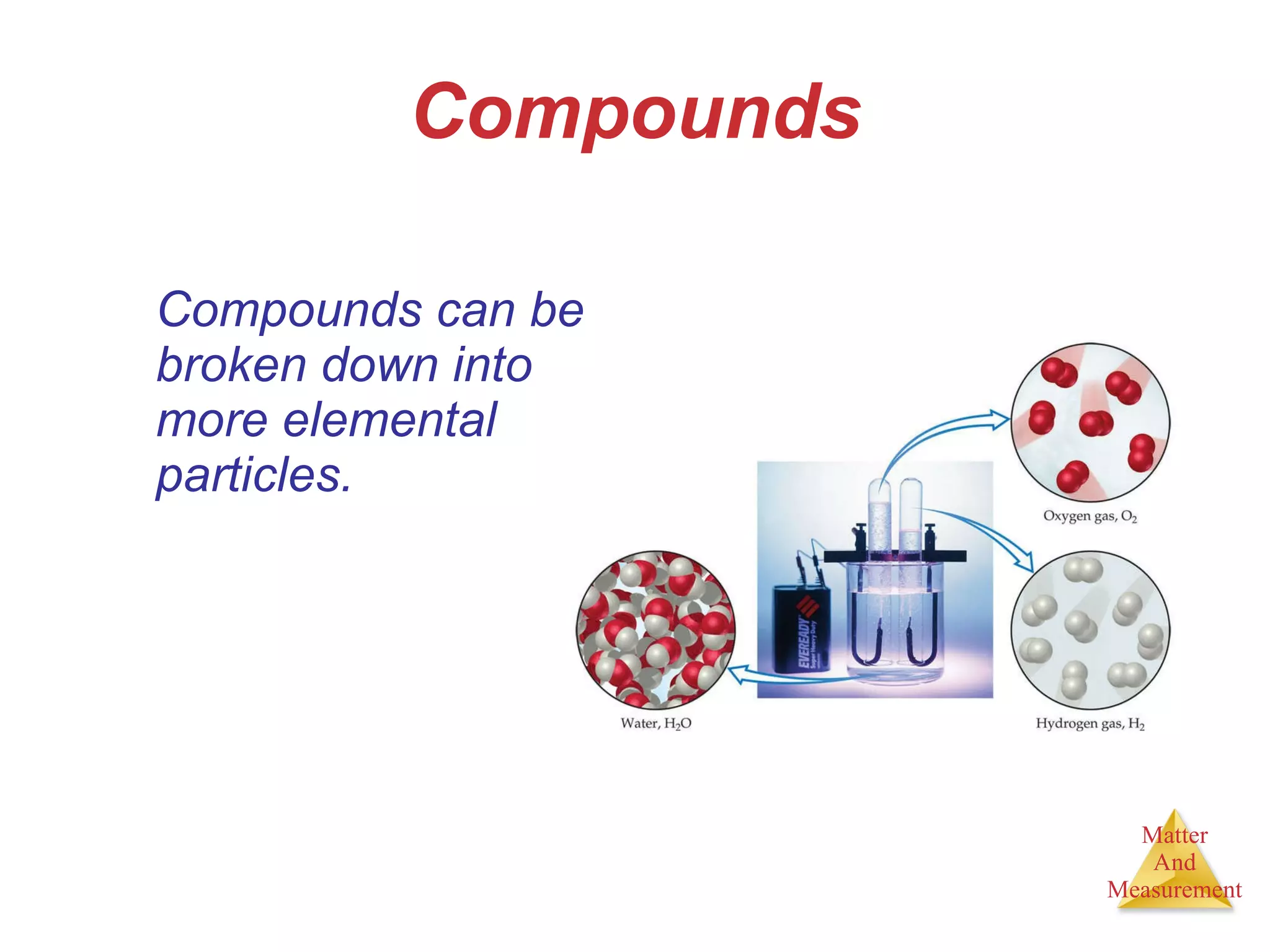
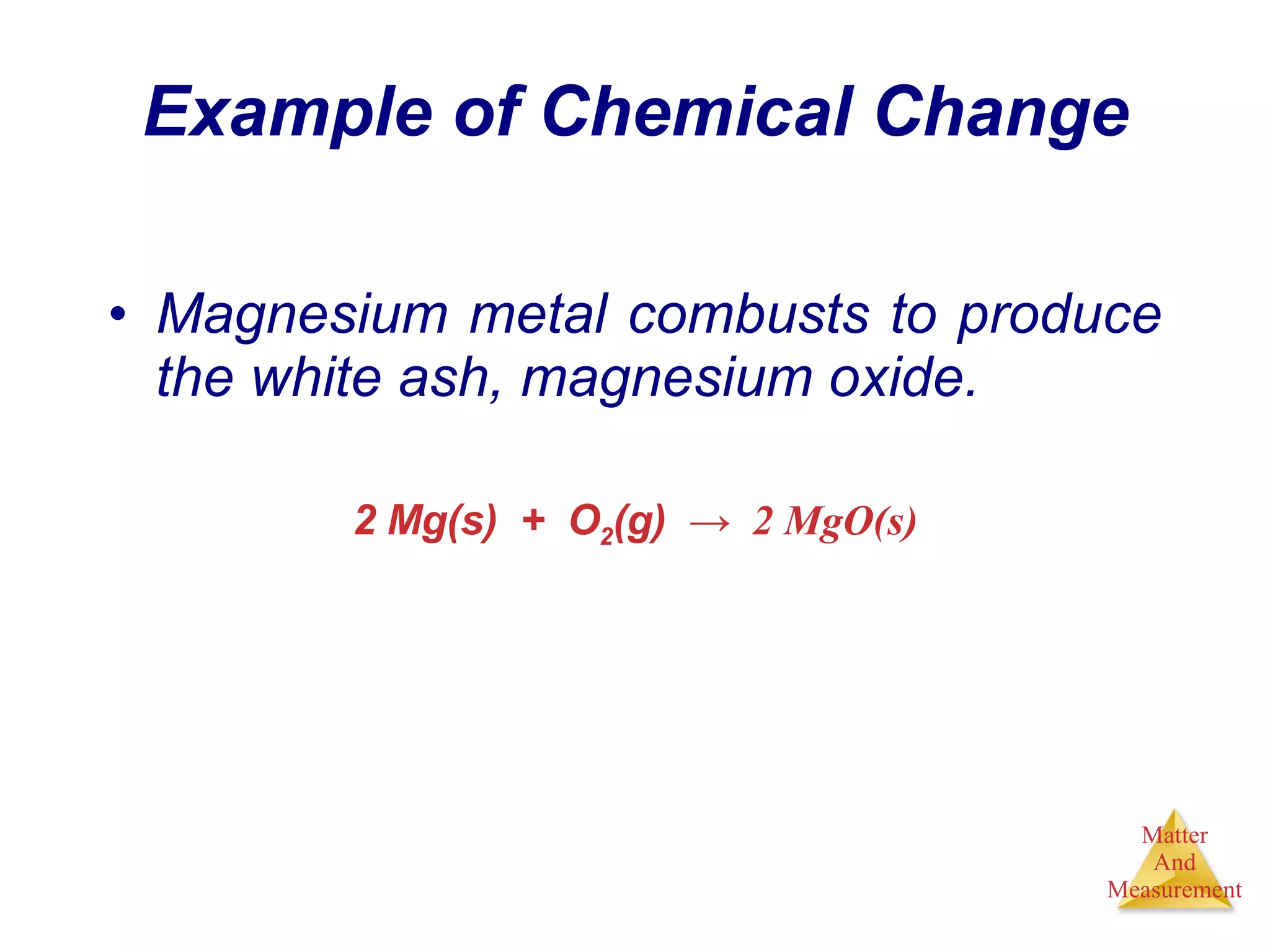
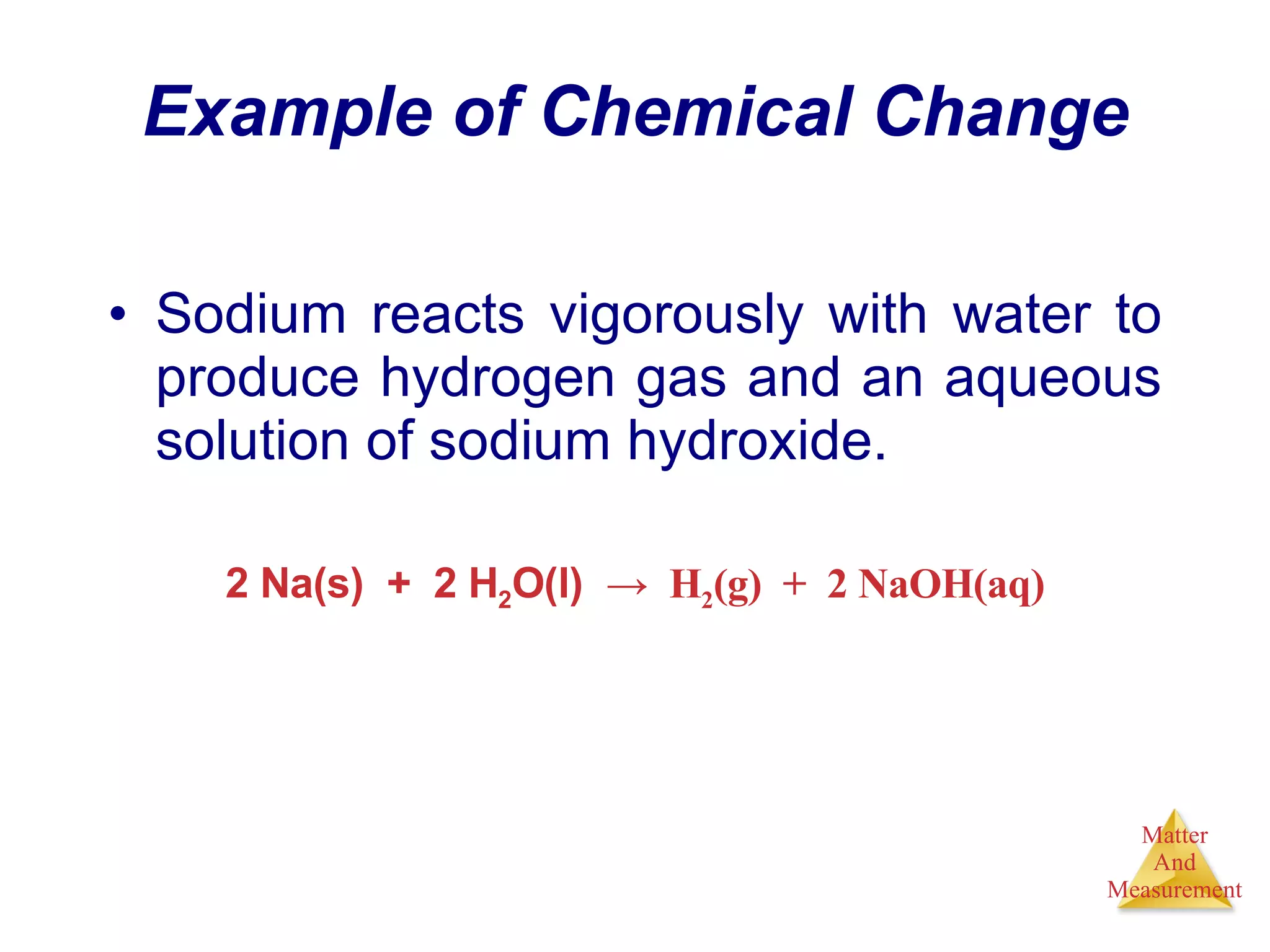
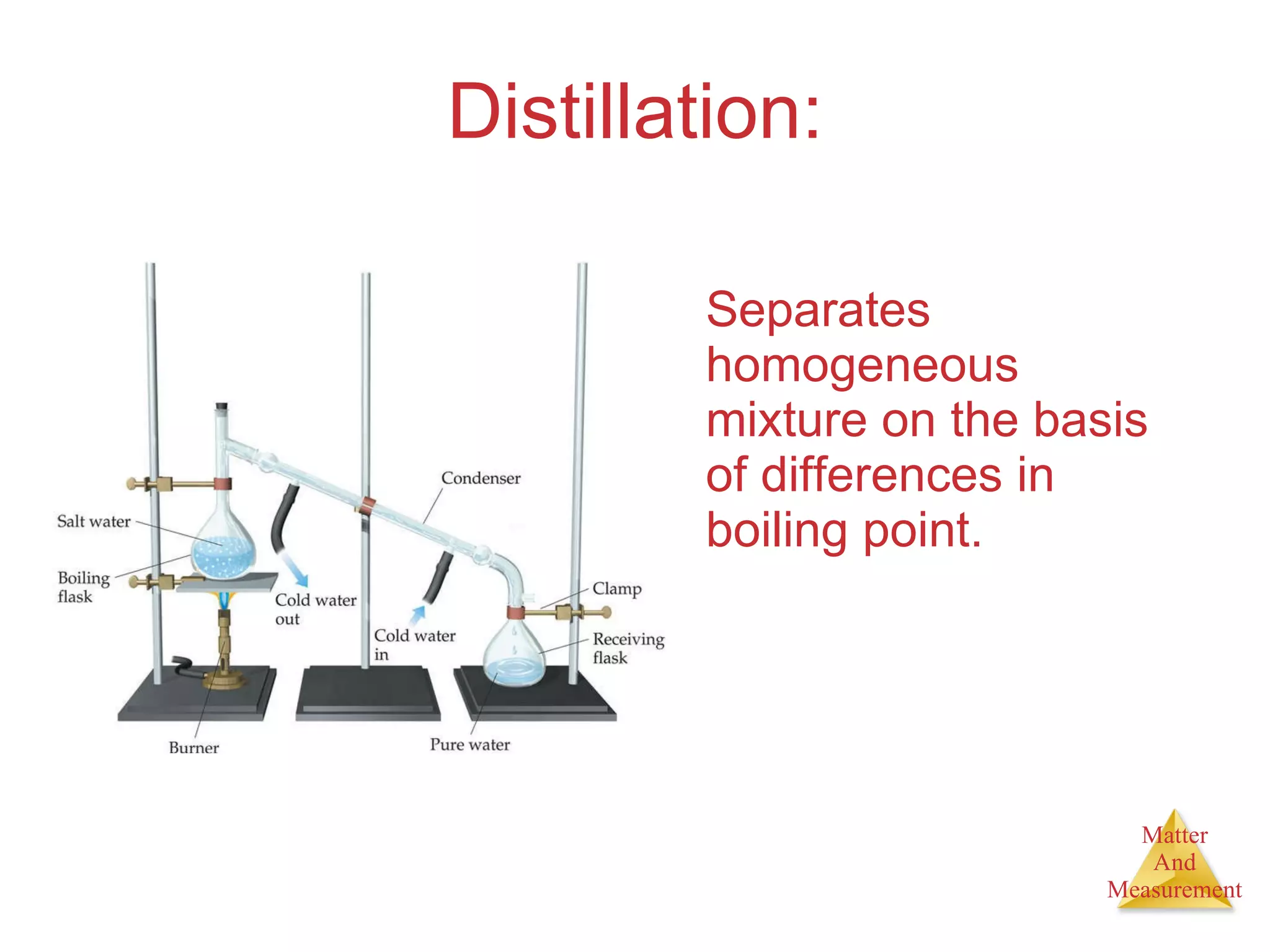
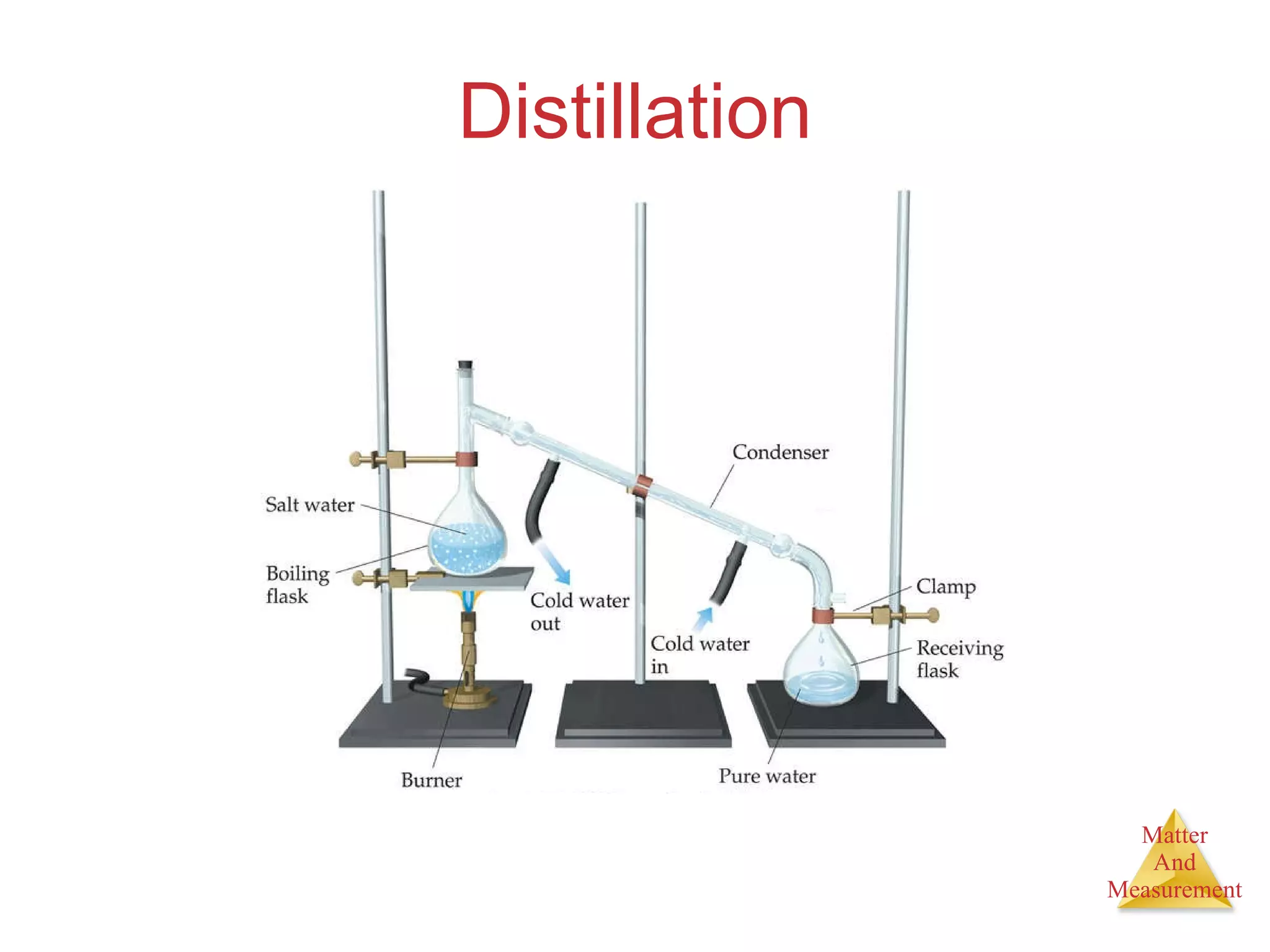
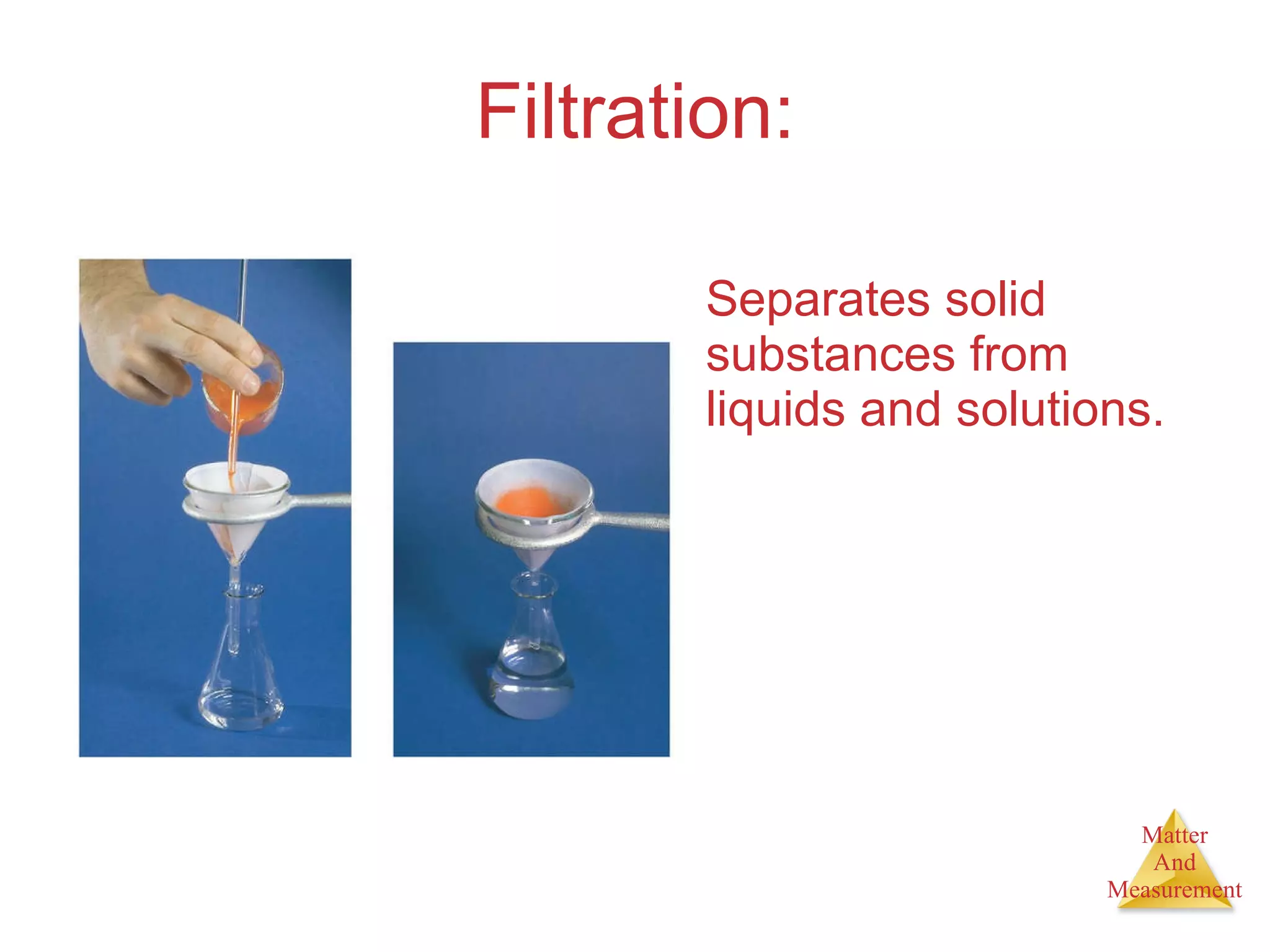
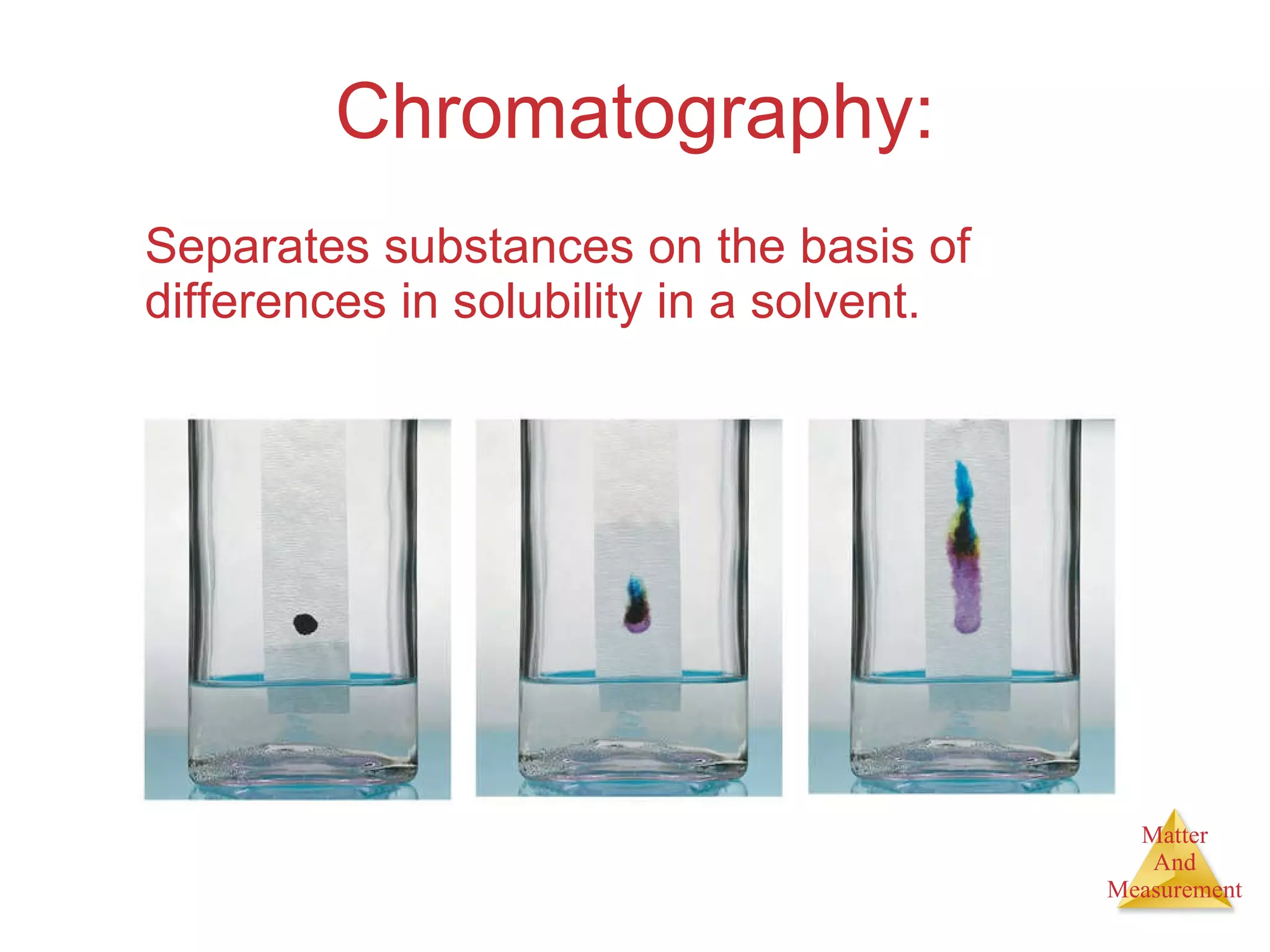
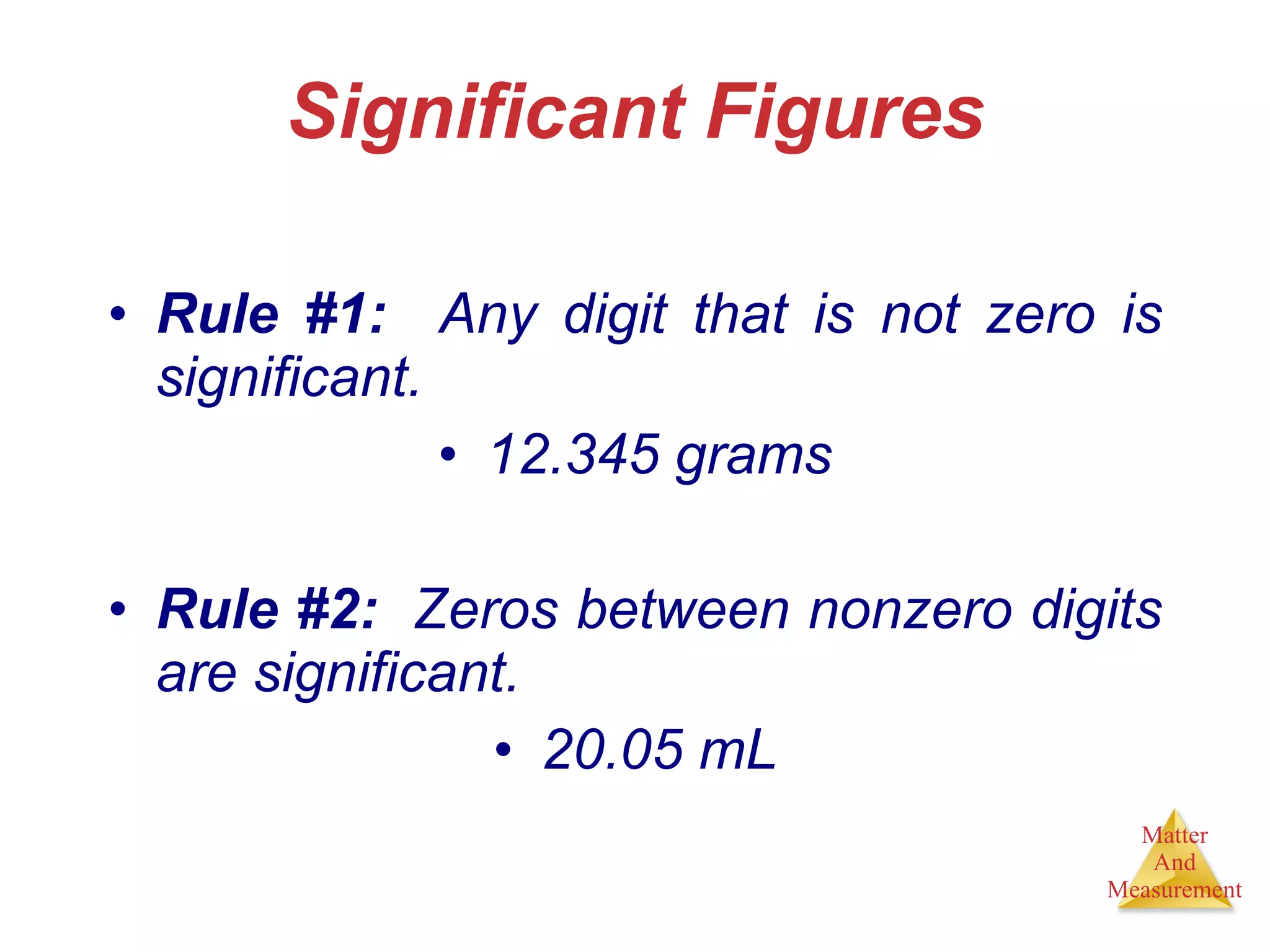
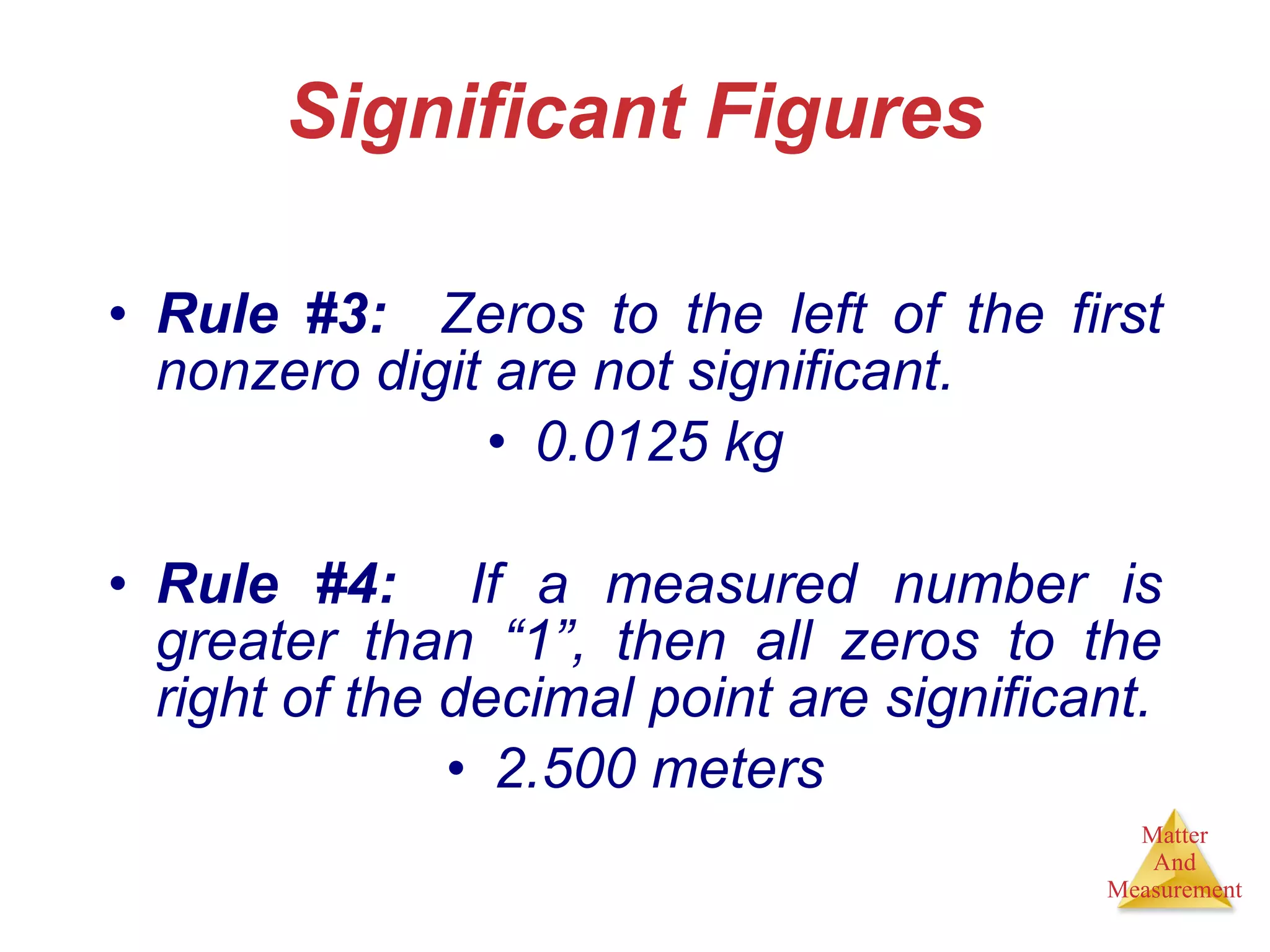
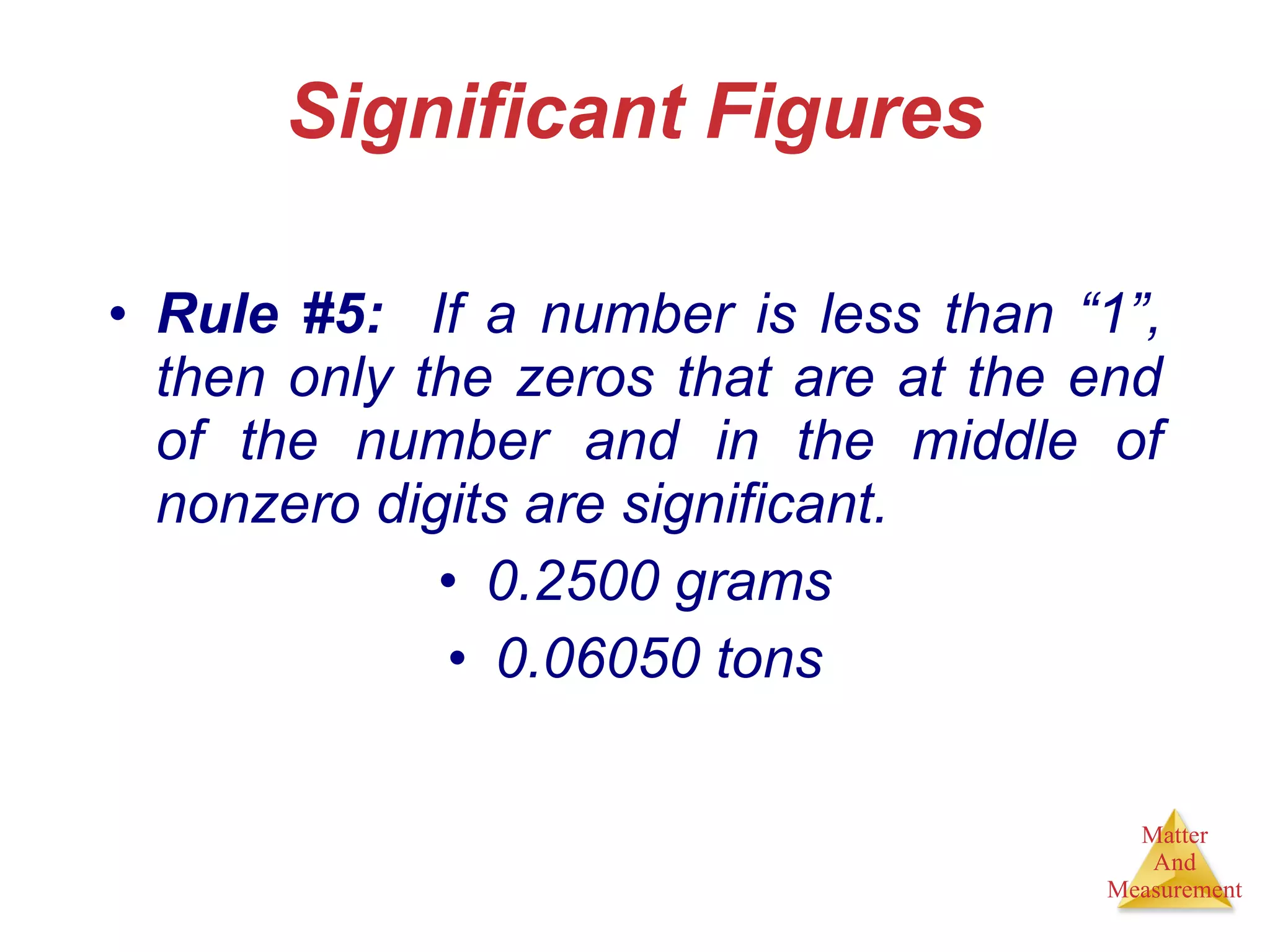
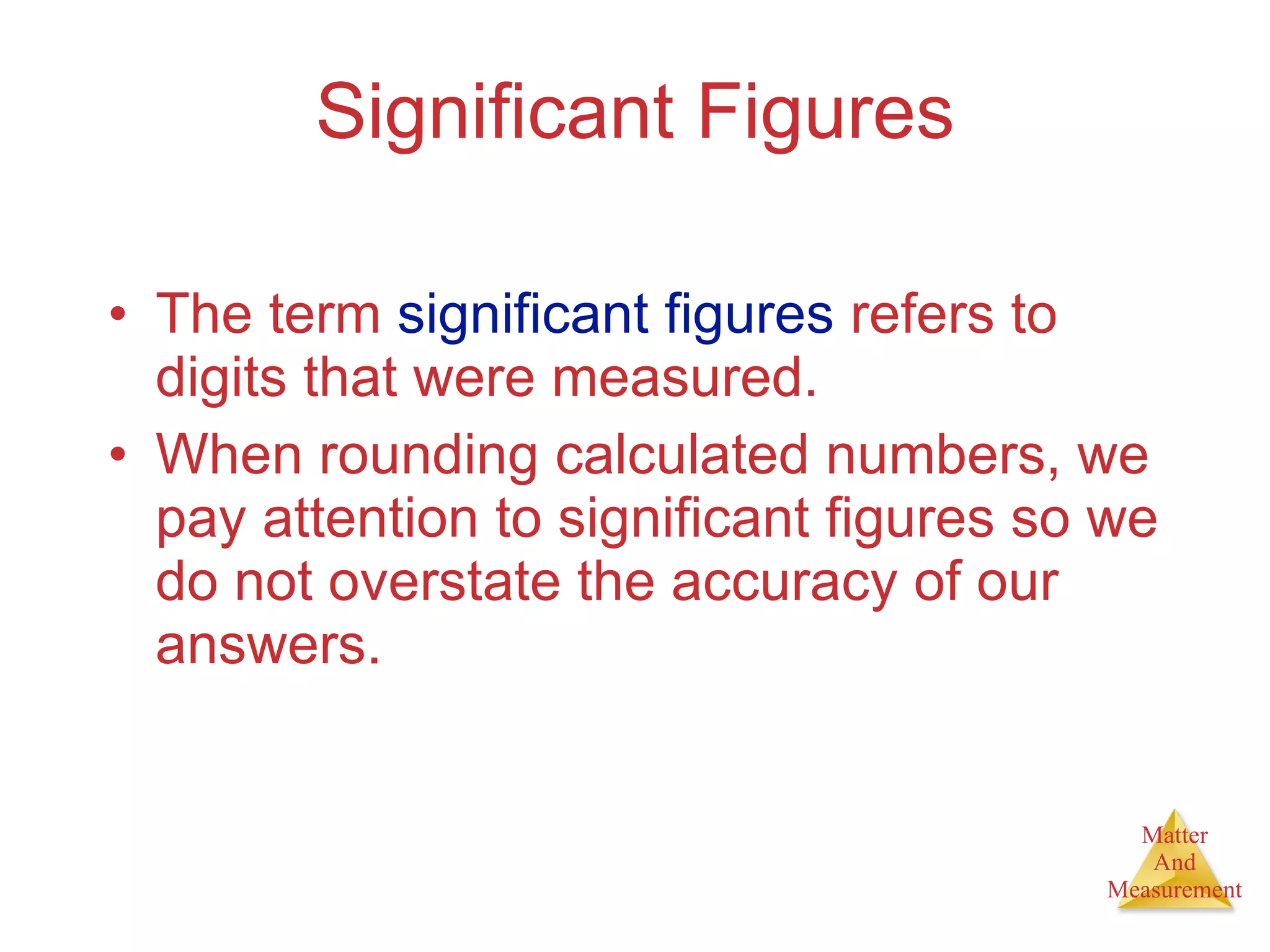
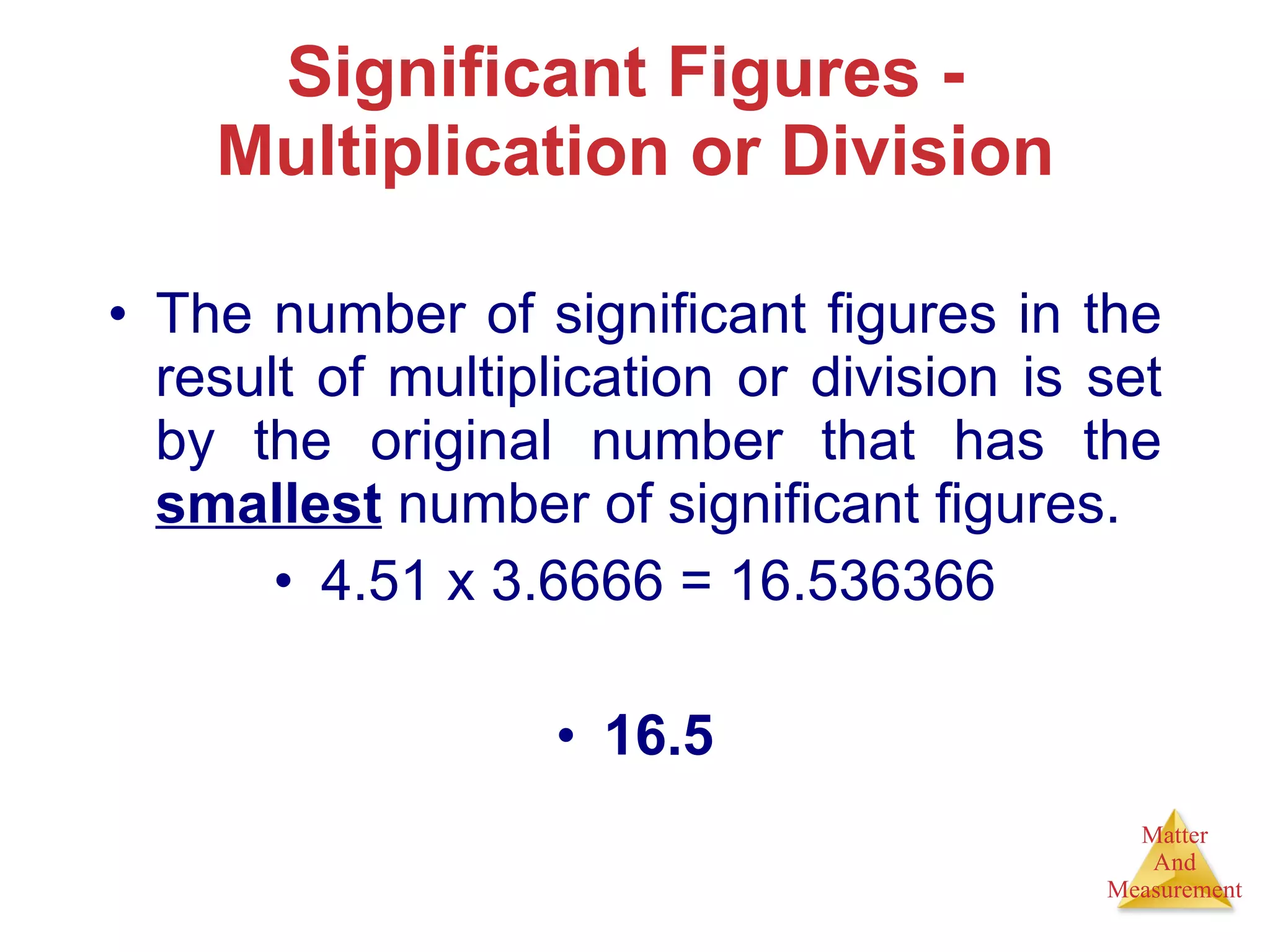
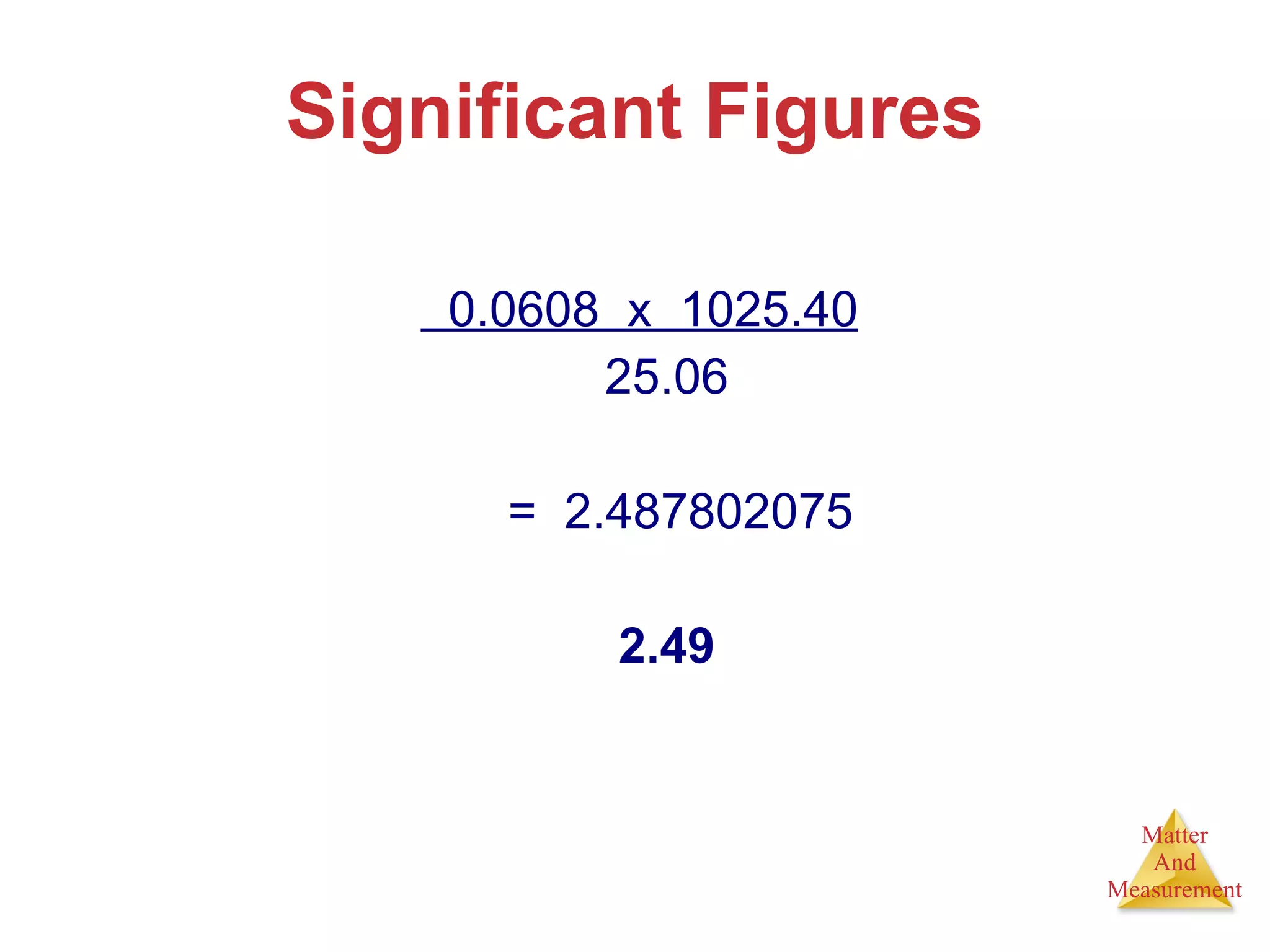
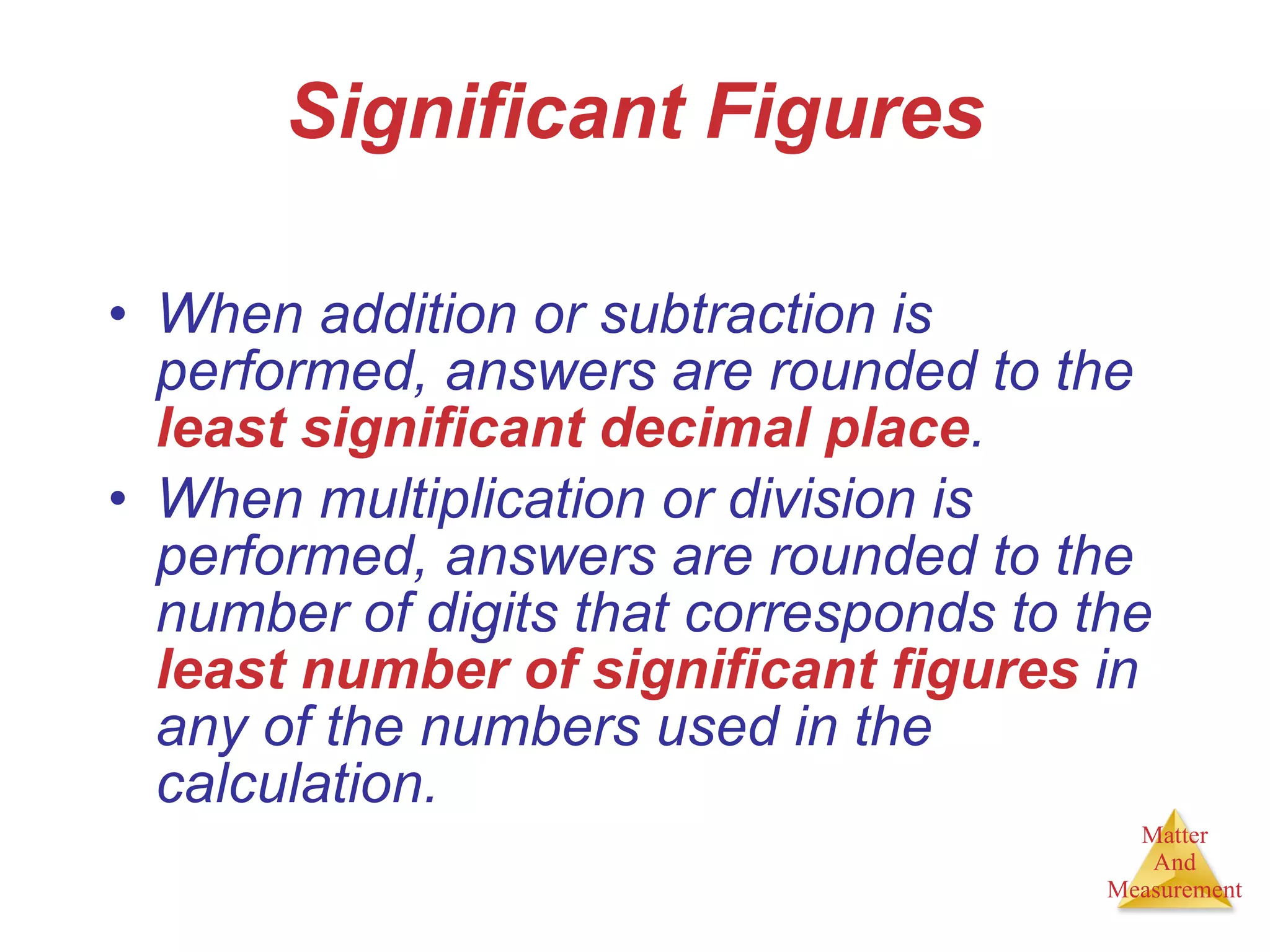
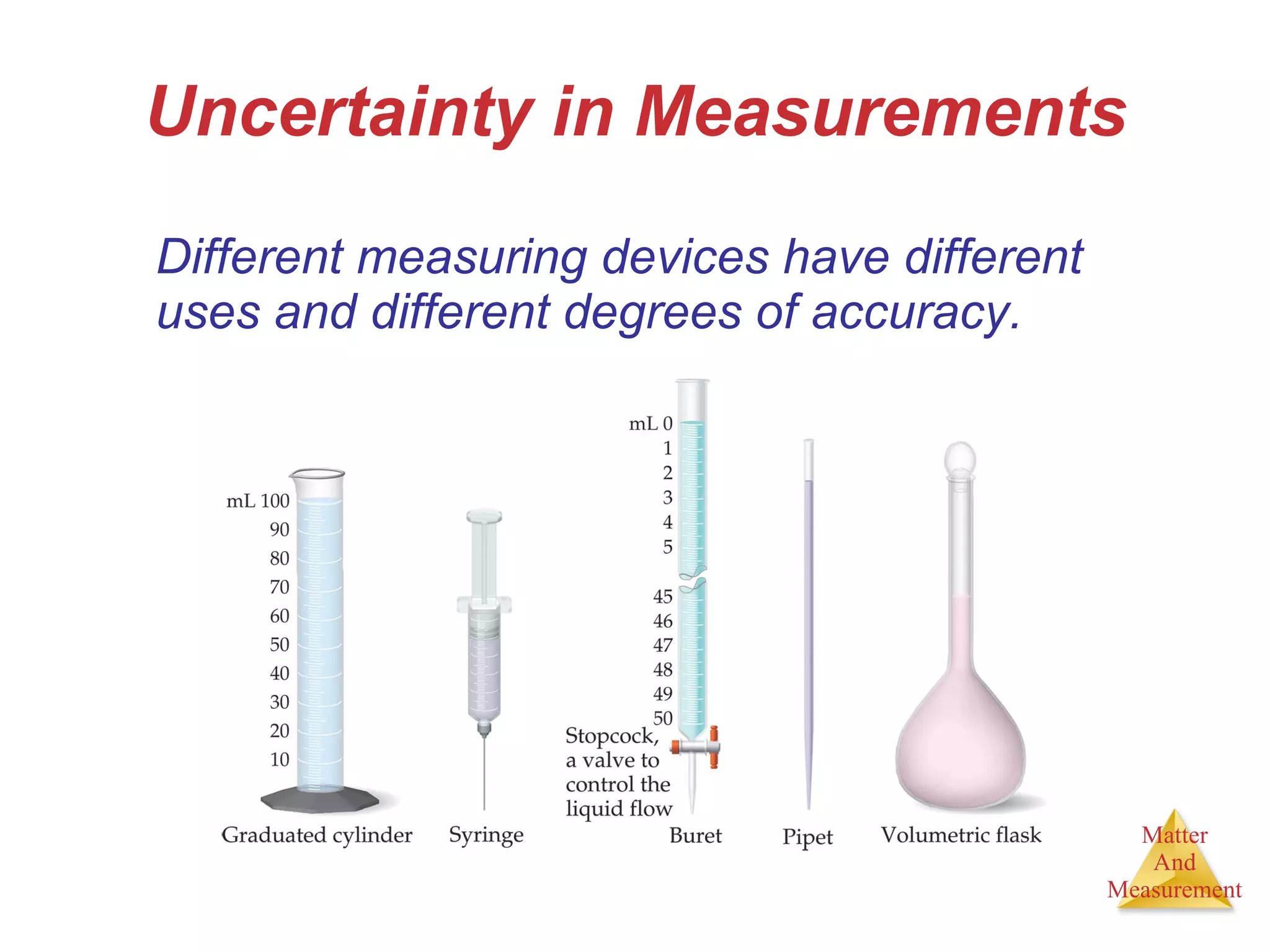
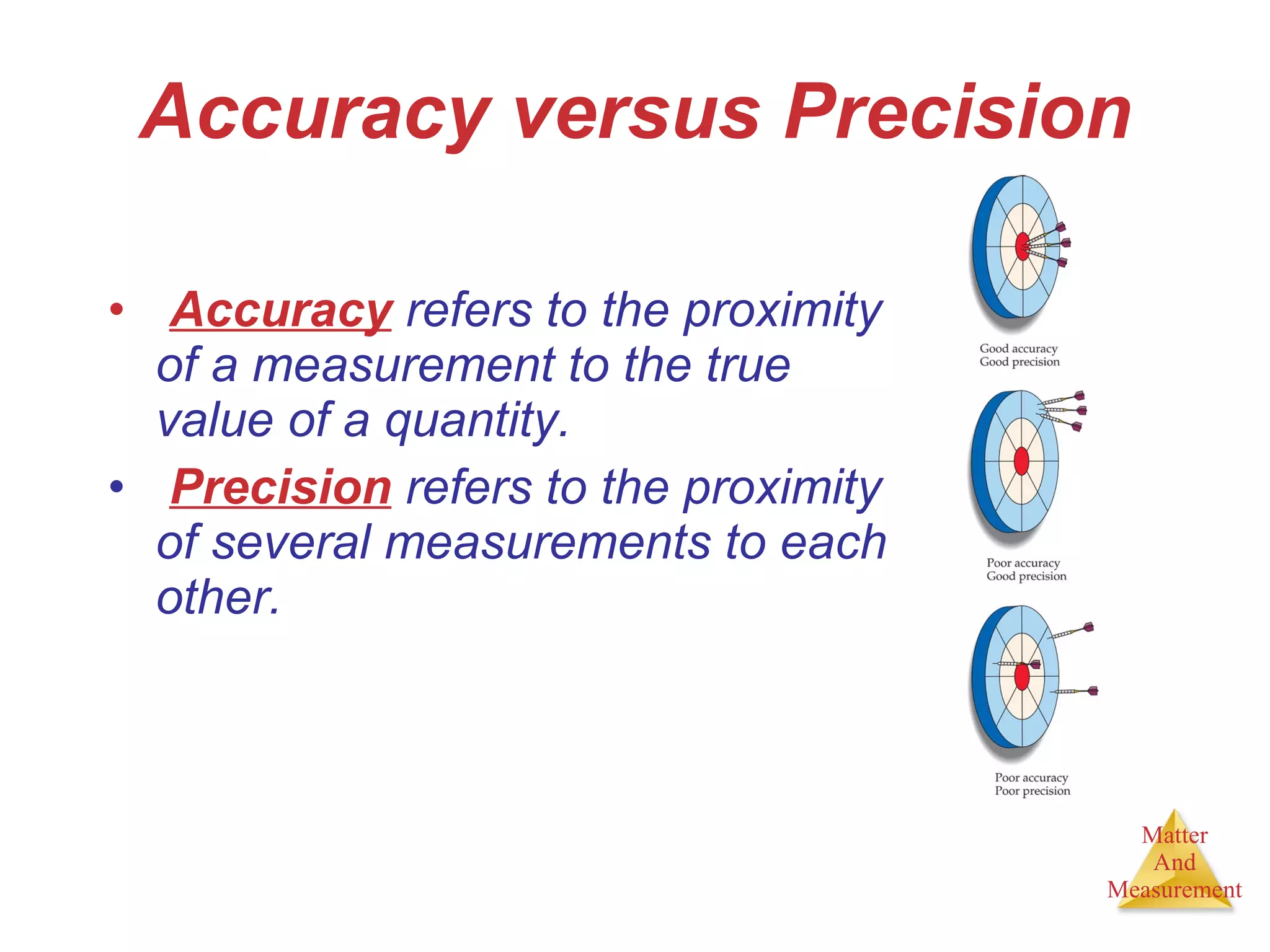
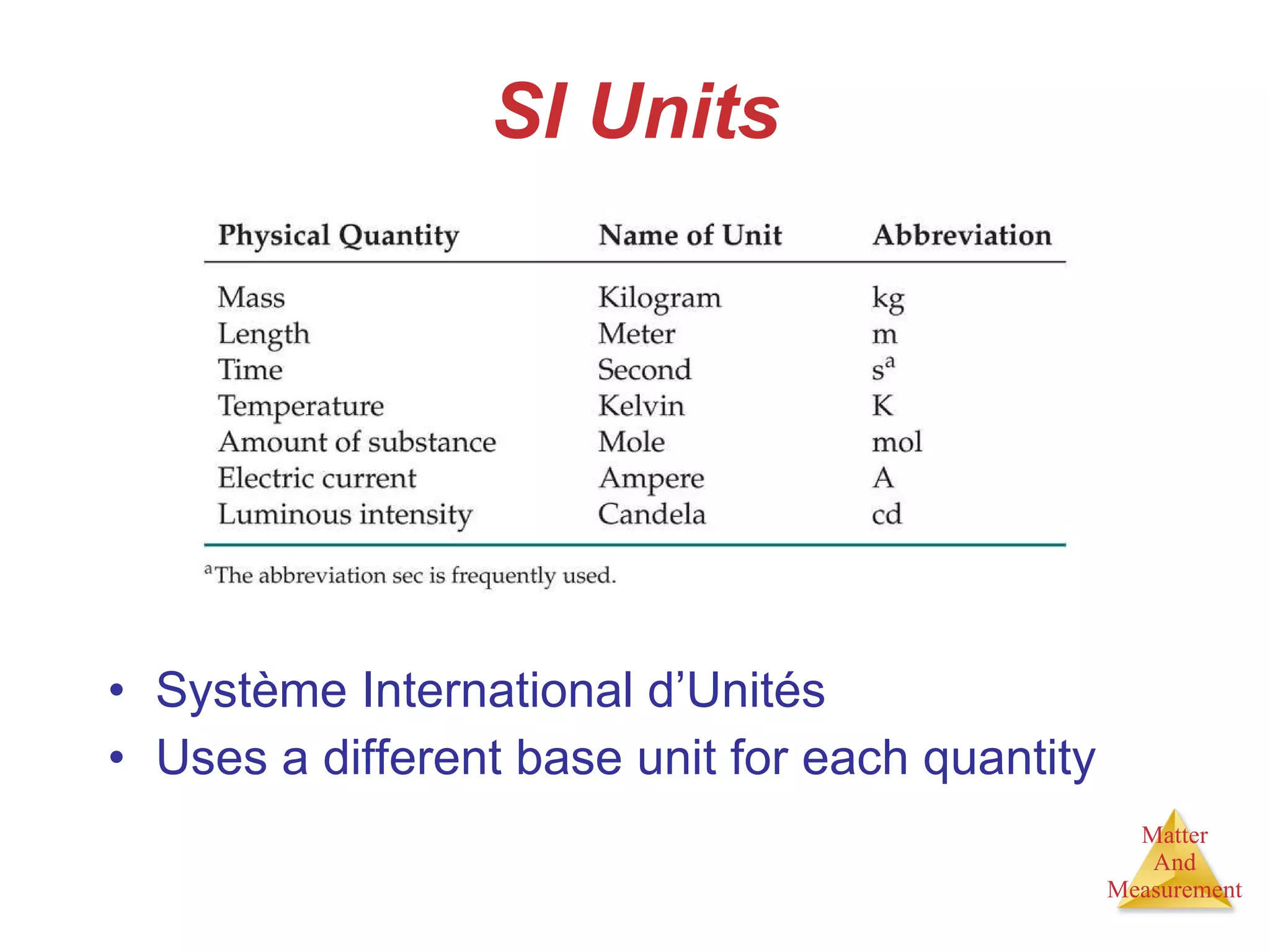
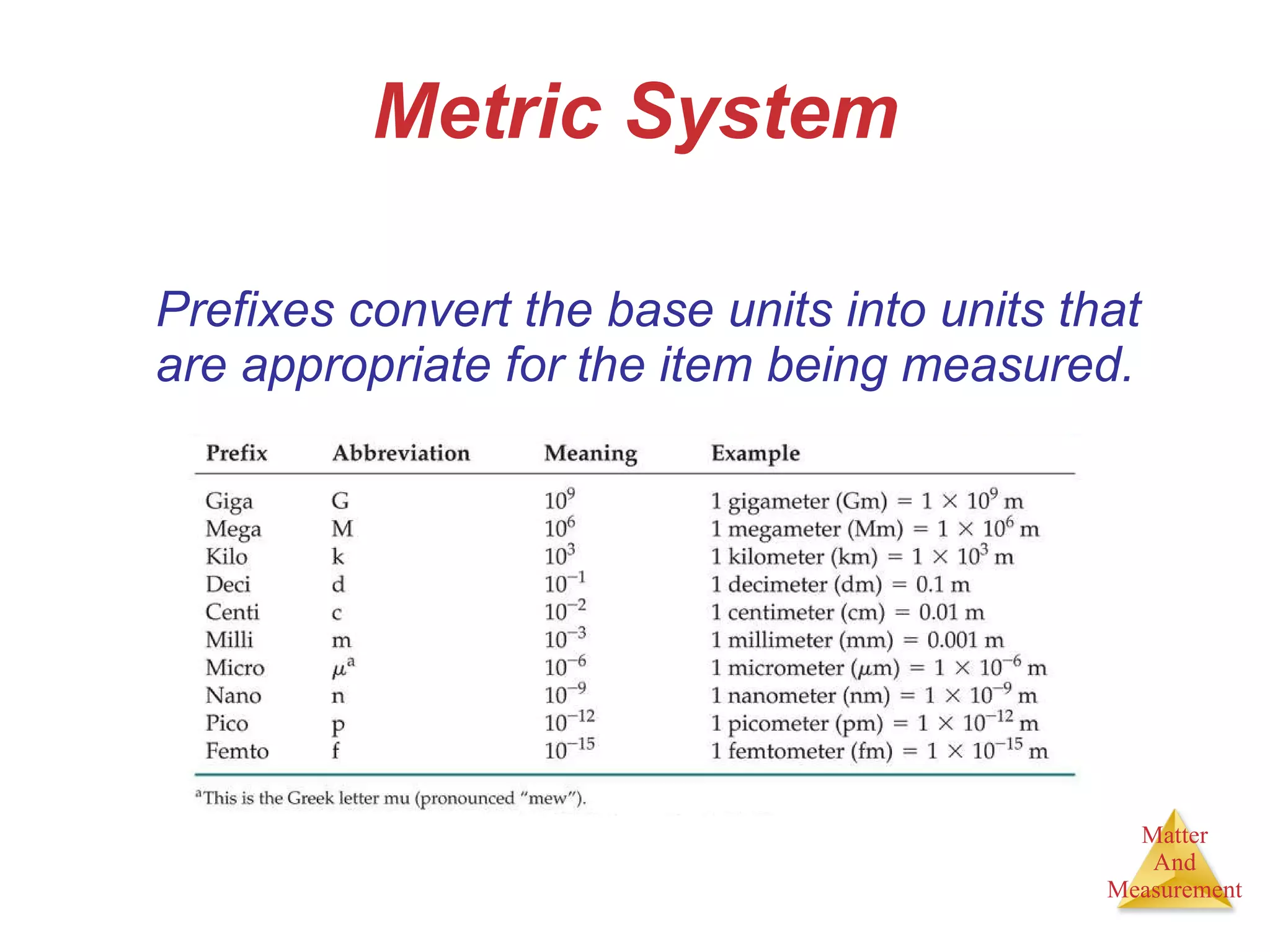
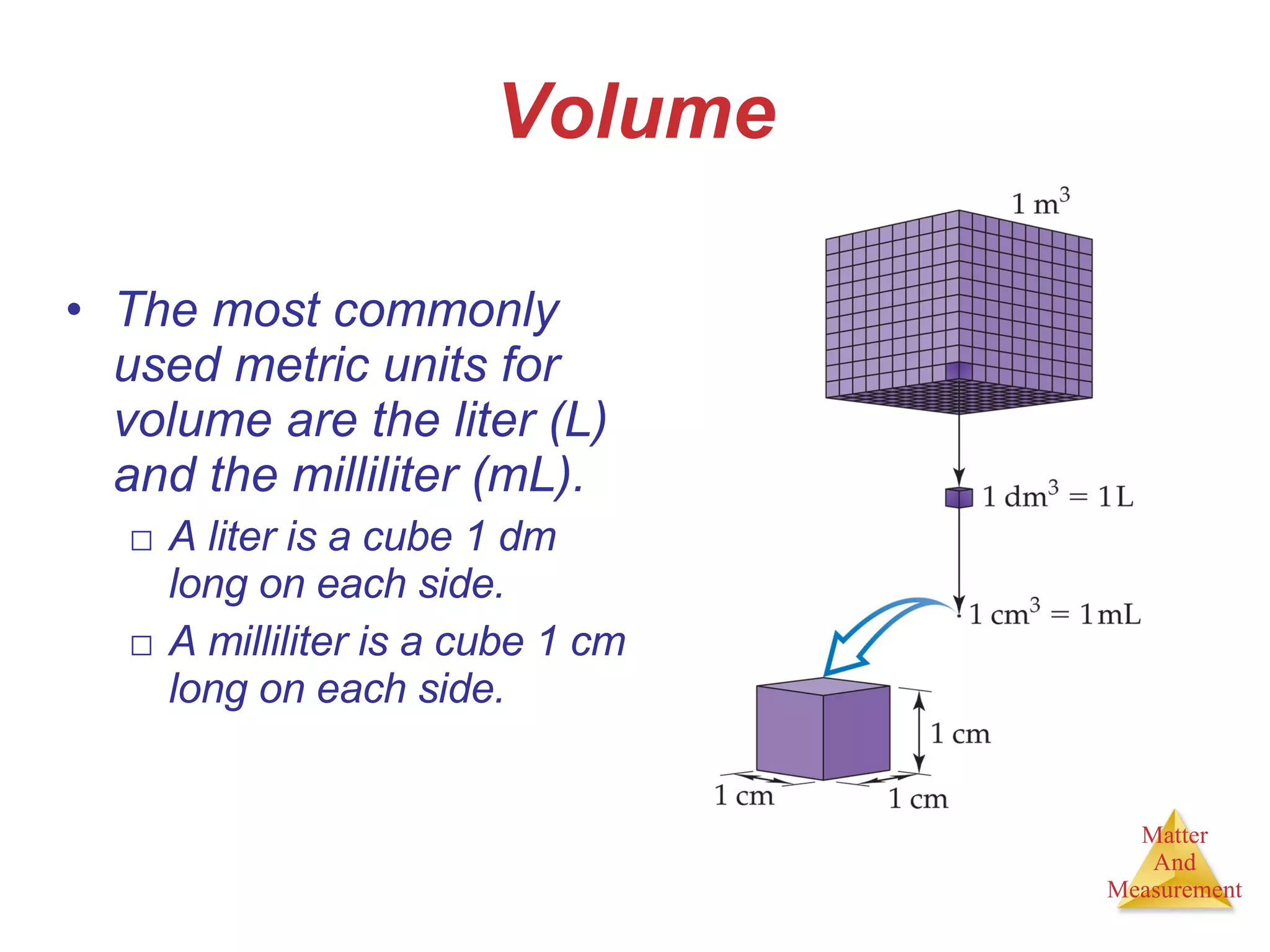
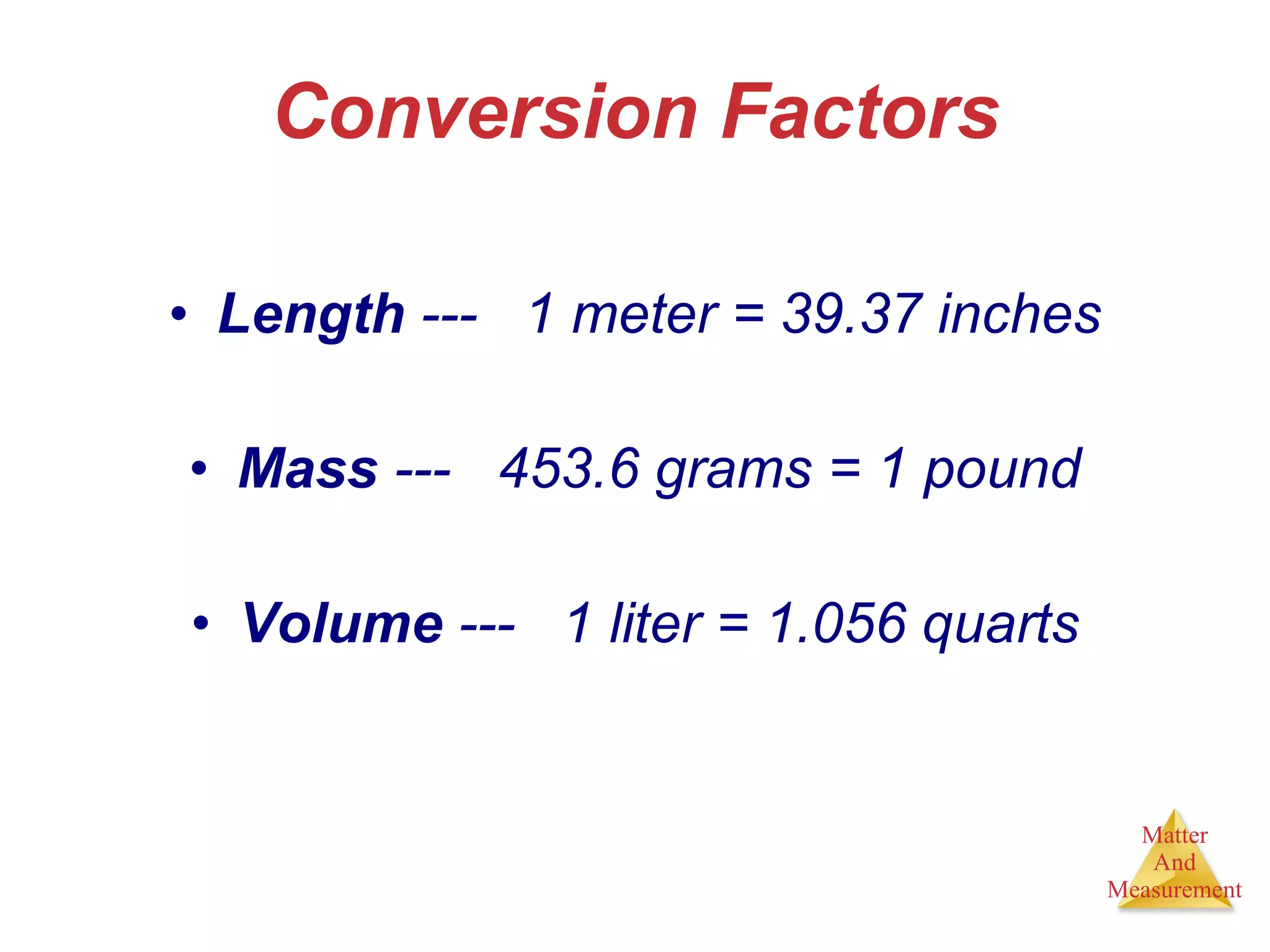
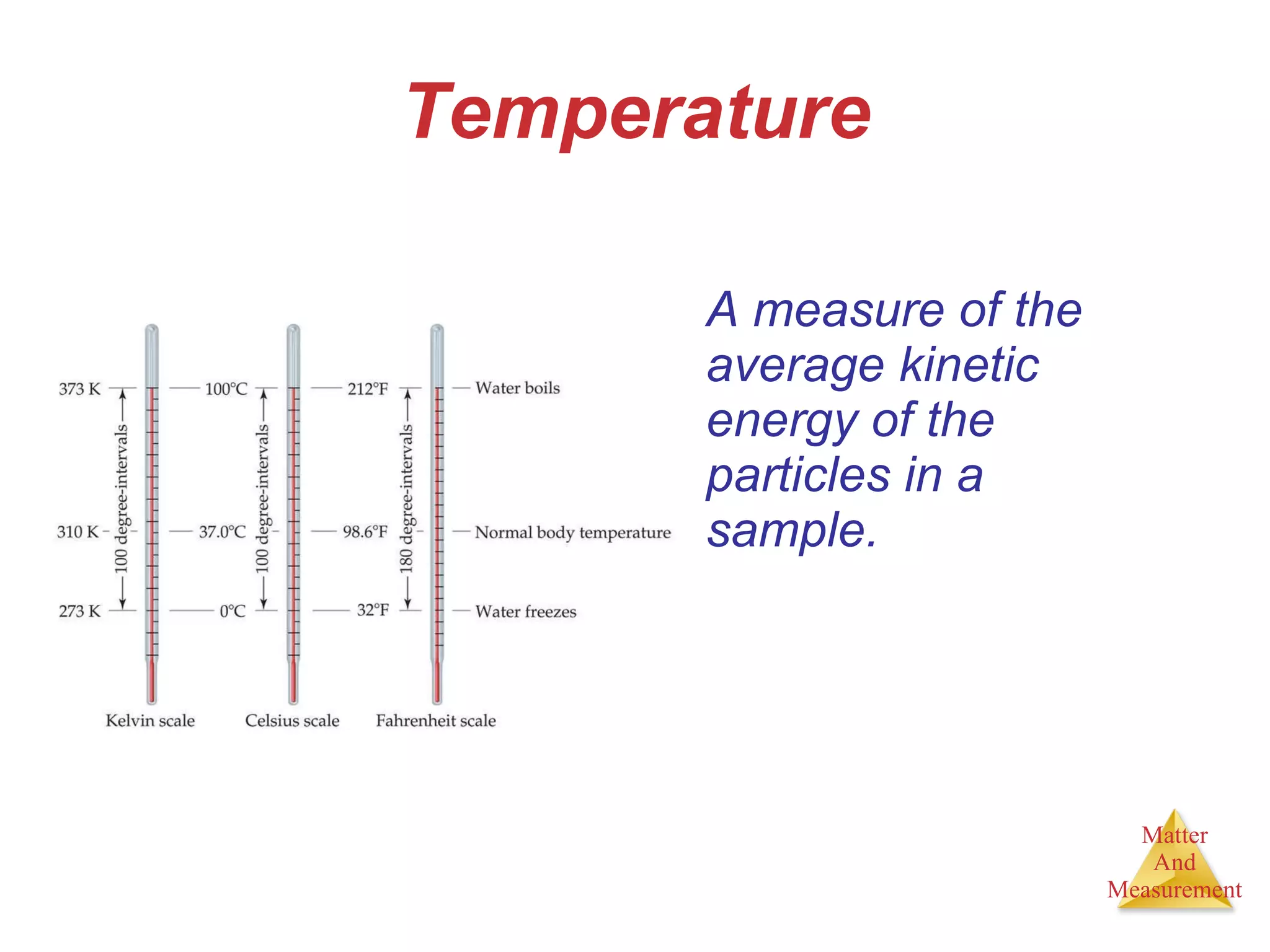
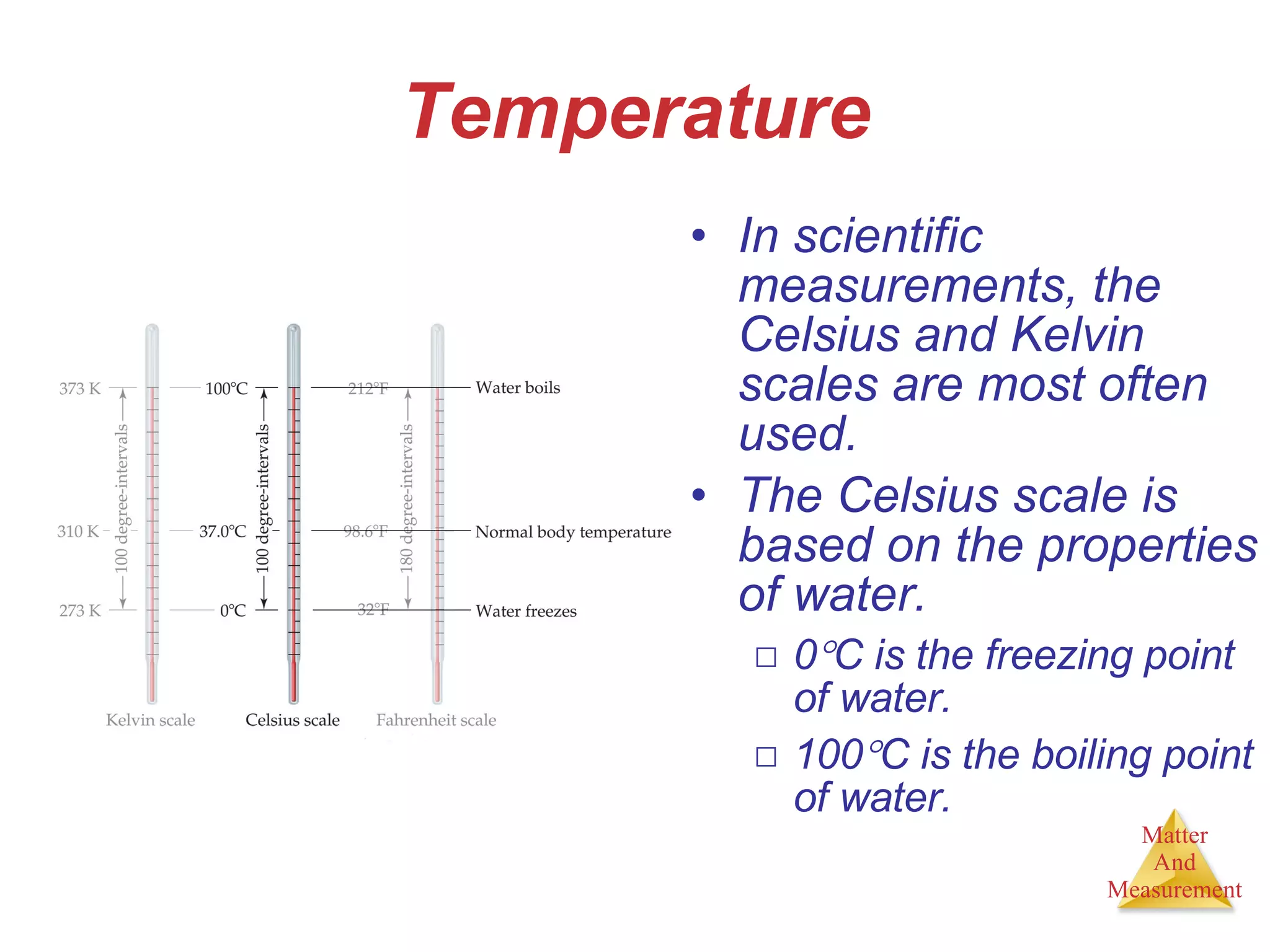
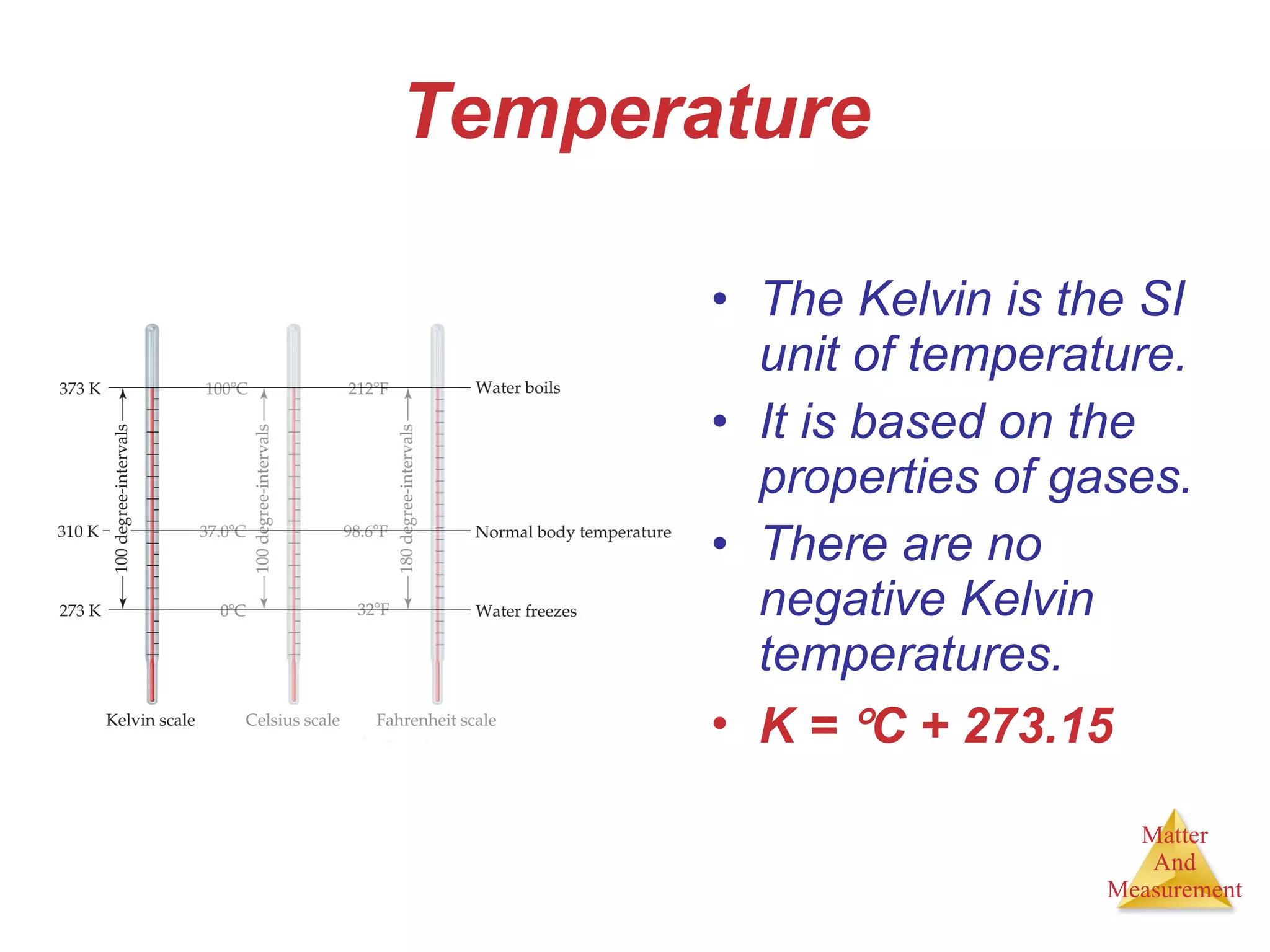
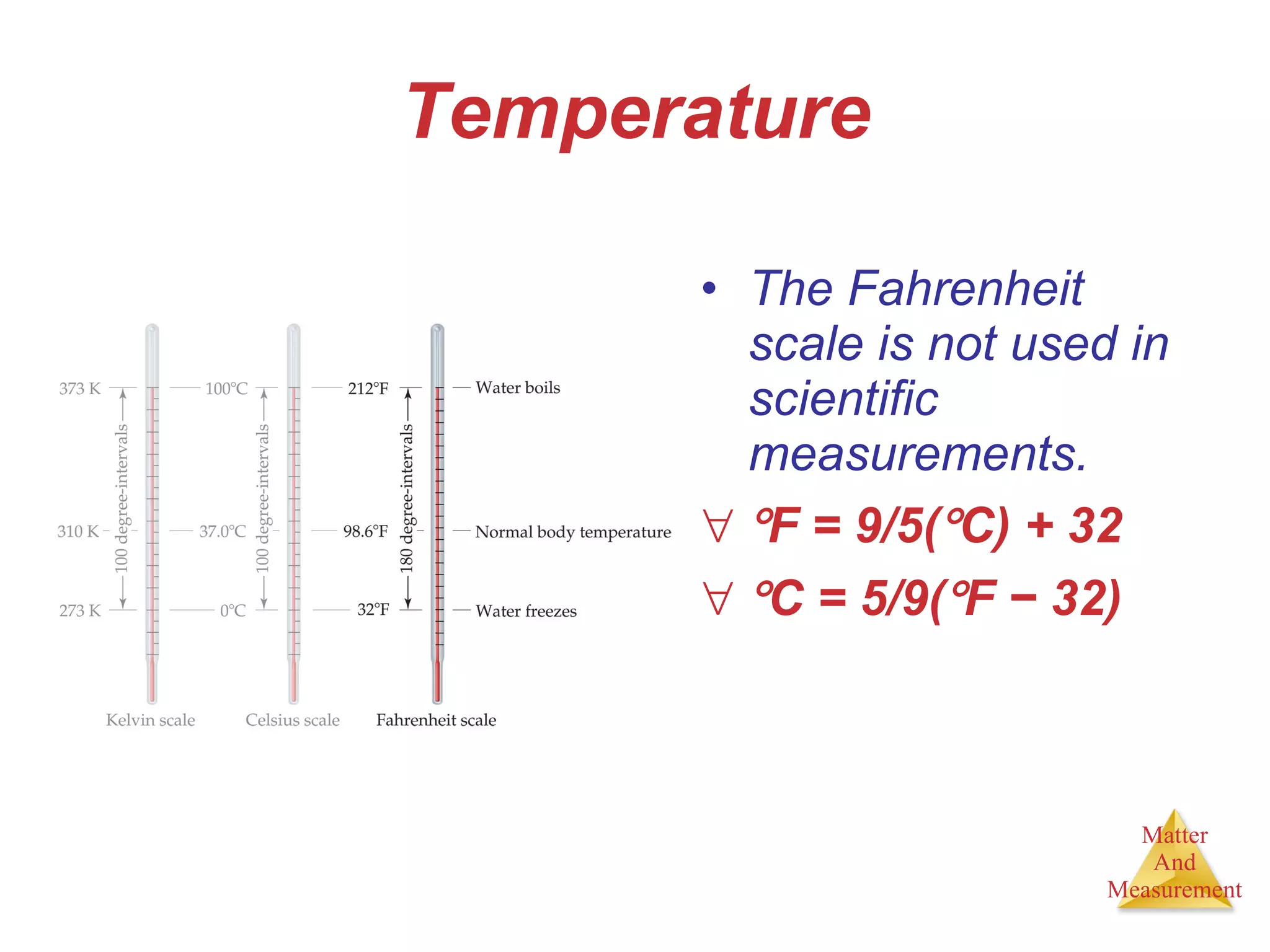
Chemistry is the study of matter, its properties, and the changes it undergoes. Matter is anything that has mass and takes up space, and is composed of atoms. Atoms are the building blocks of matter and each element is made of the same type of atom. Compounds are made of two or more different elements chemically bonded together. Mixtures contain two or more substances mixed but not chemically combined. Measurements in chemistry use significant figures and the SI system of units including meters, grams, and liters.