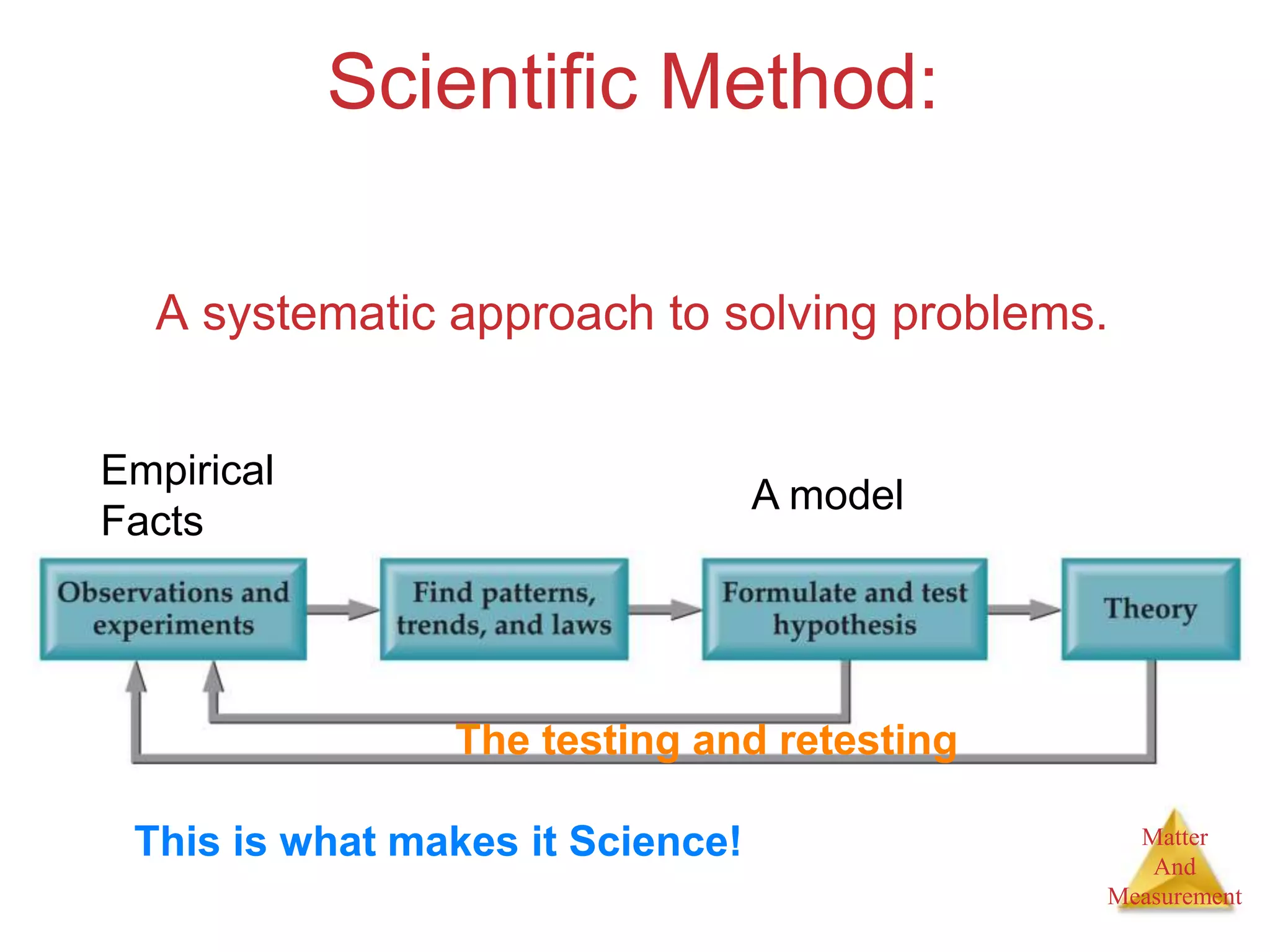
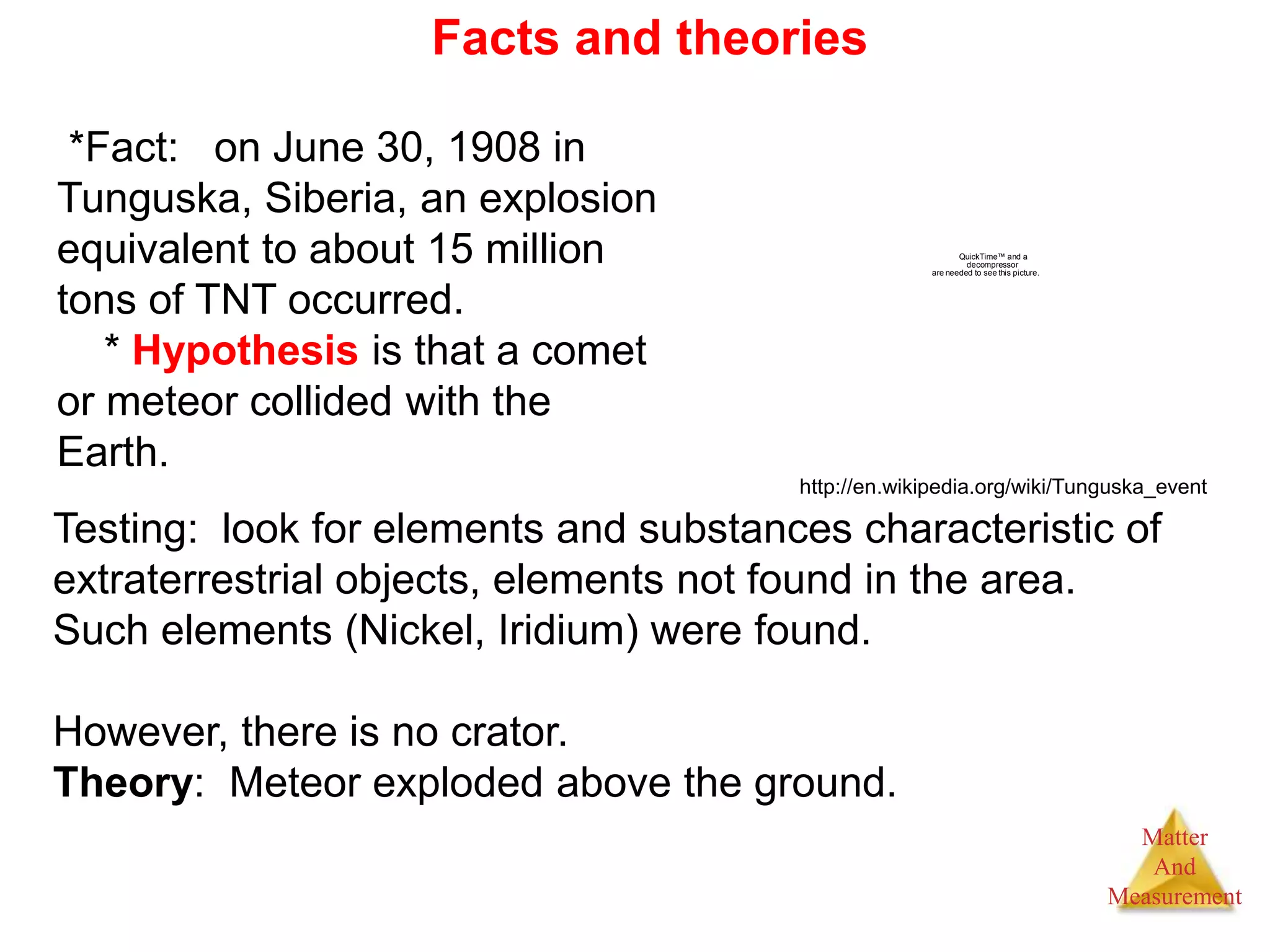
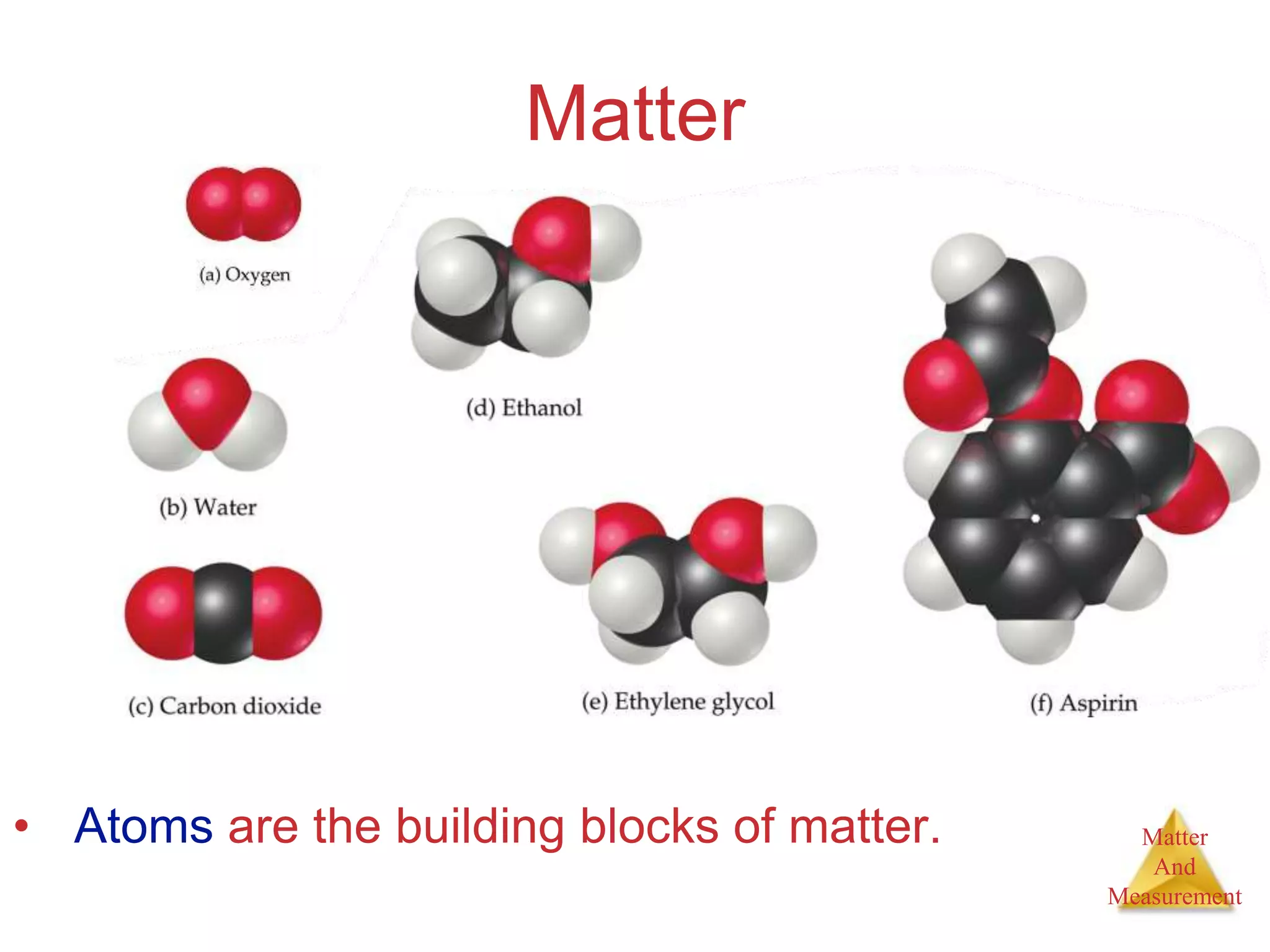
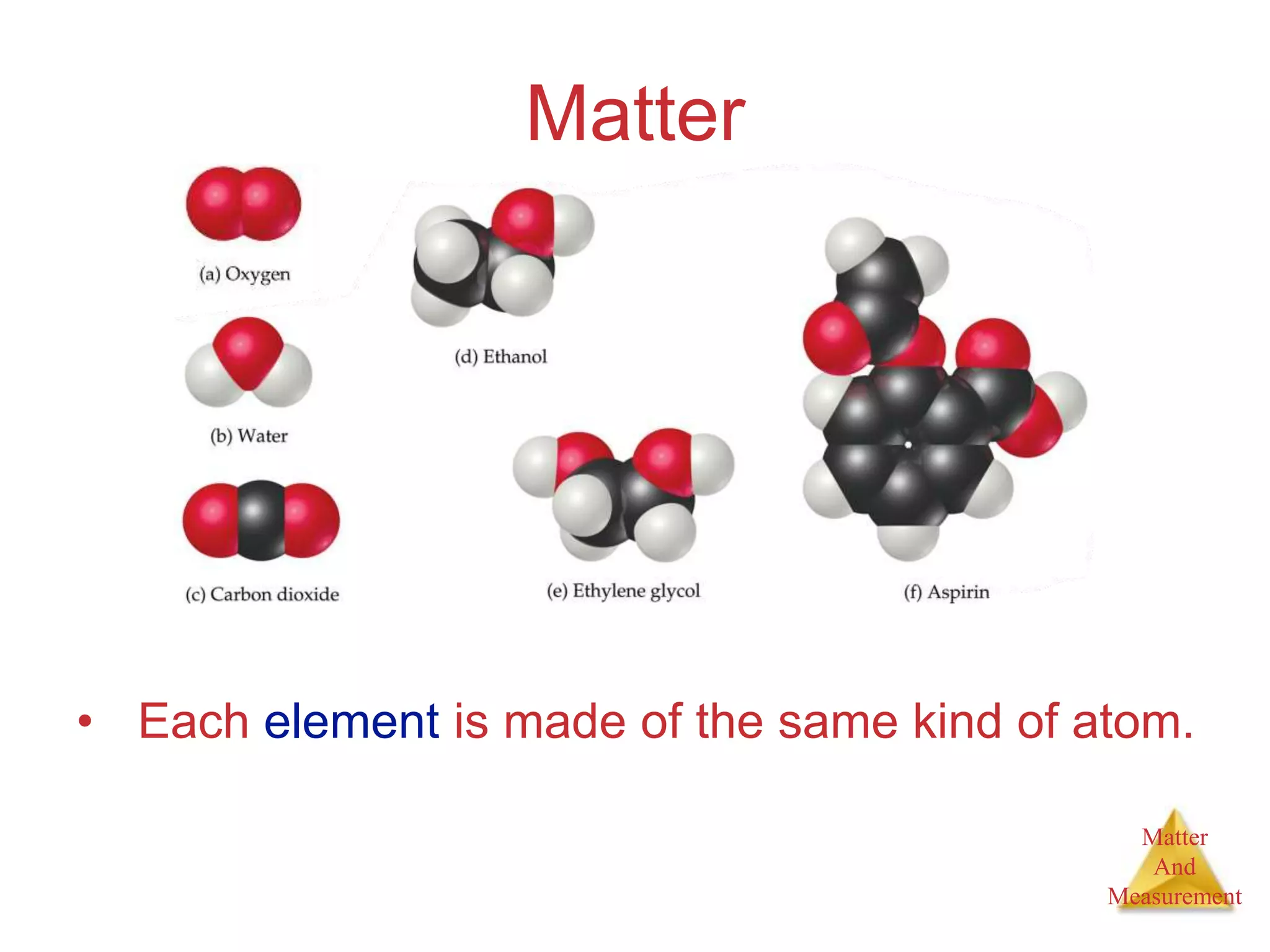
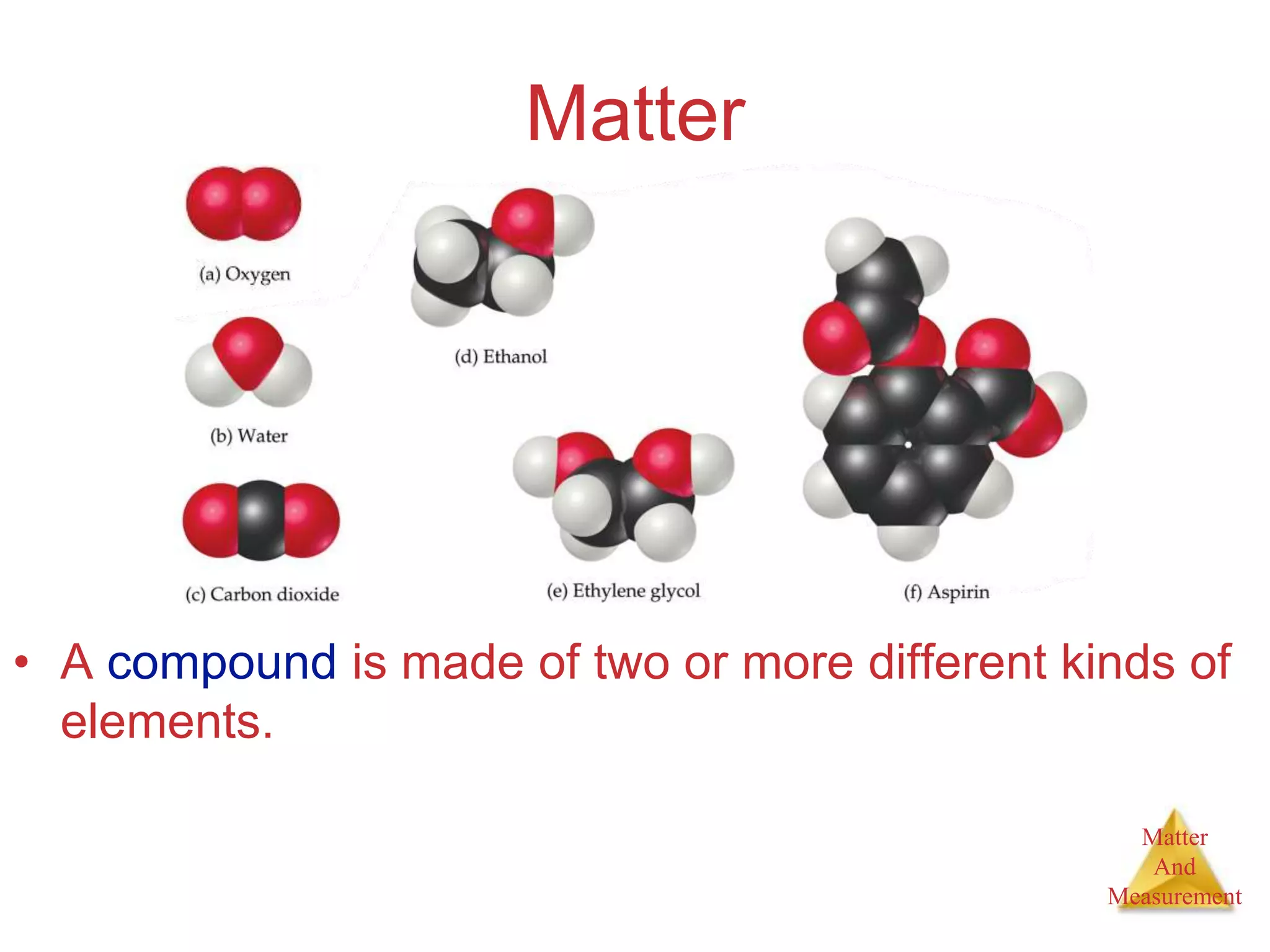
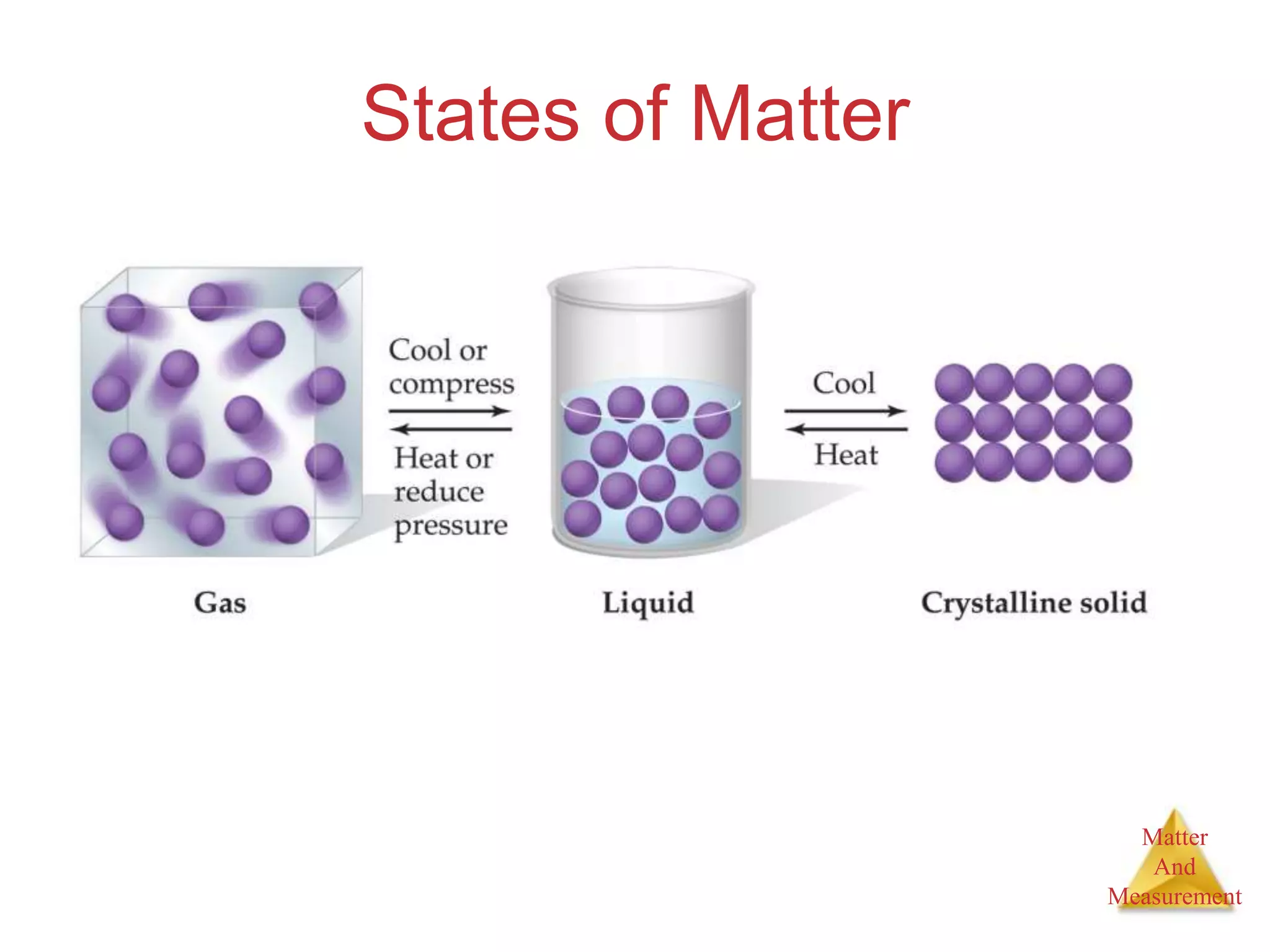
1. The document introduces topics related to matter and measurement in chemistry including the scientific method, distinguishing between facts and theories, states of matter, and properties and changes of matter.
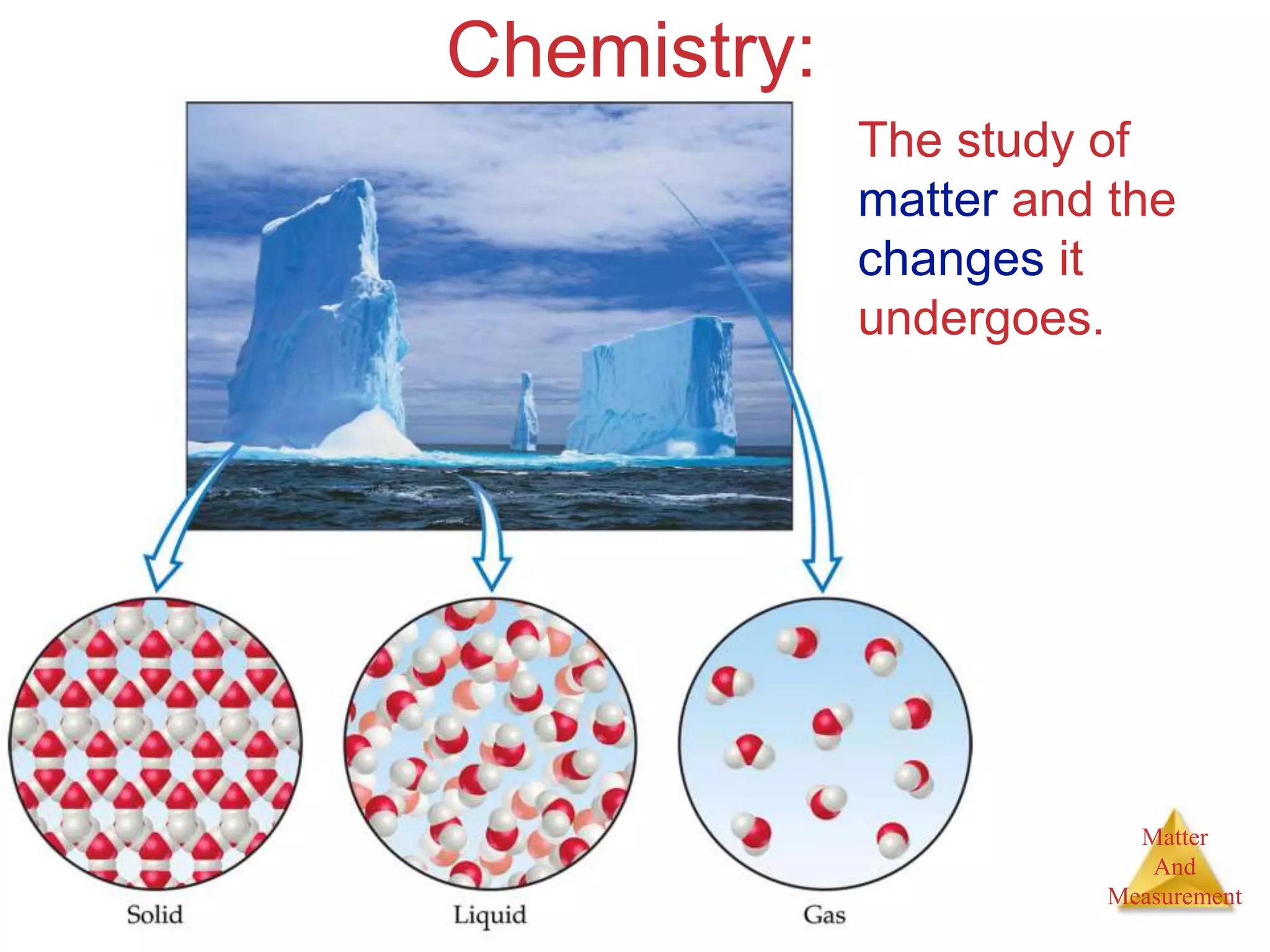
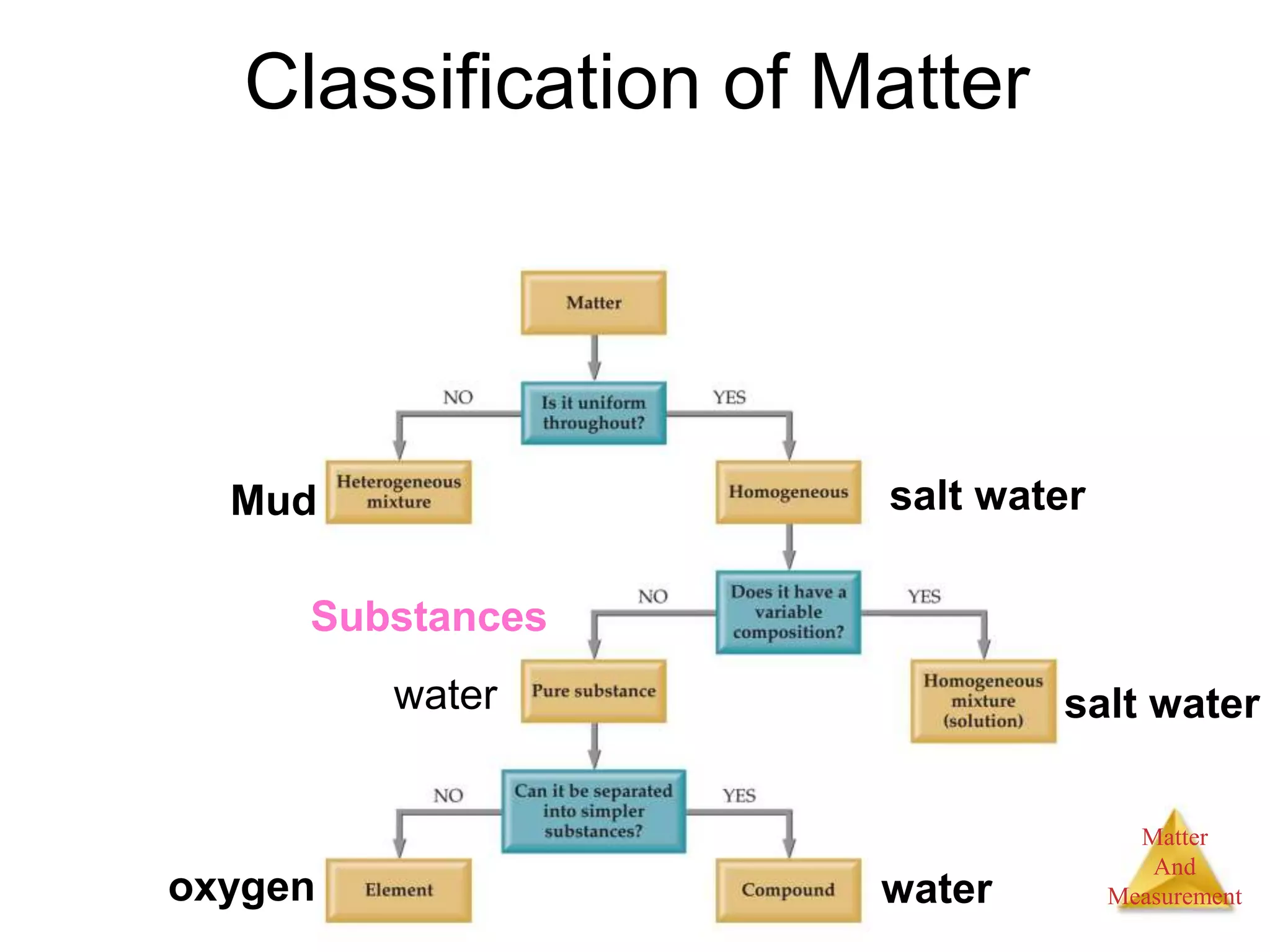
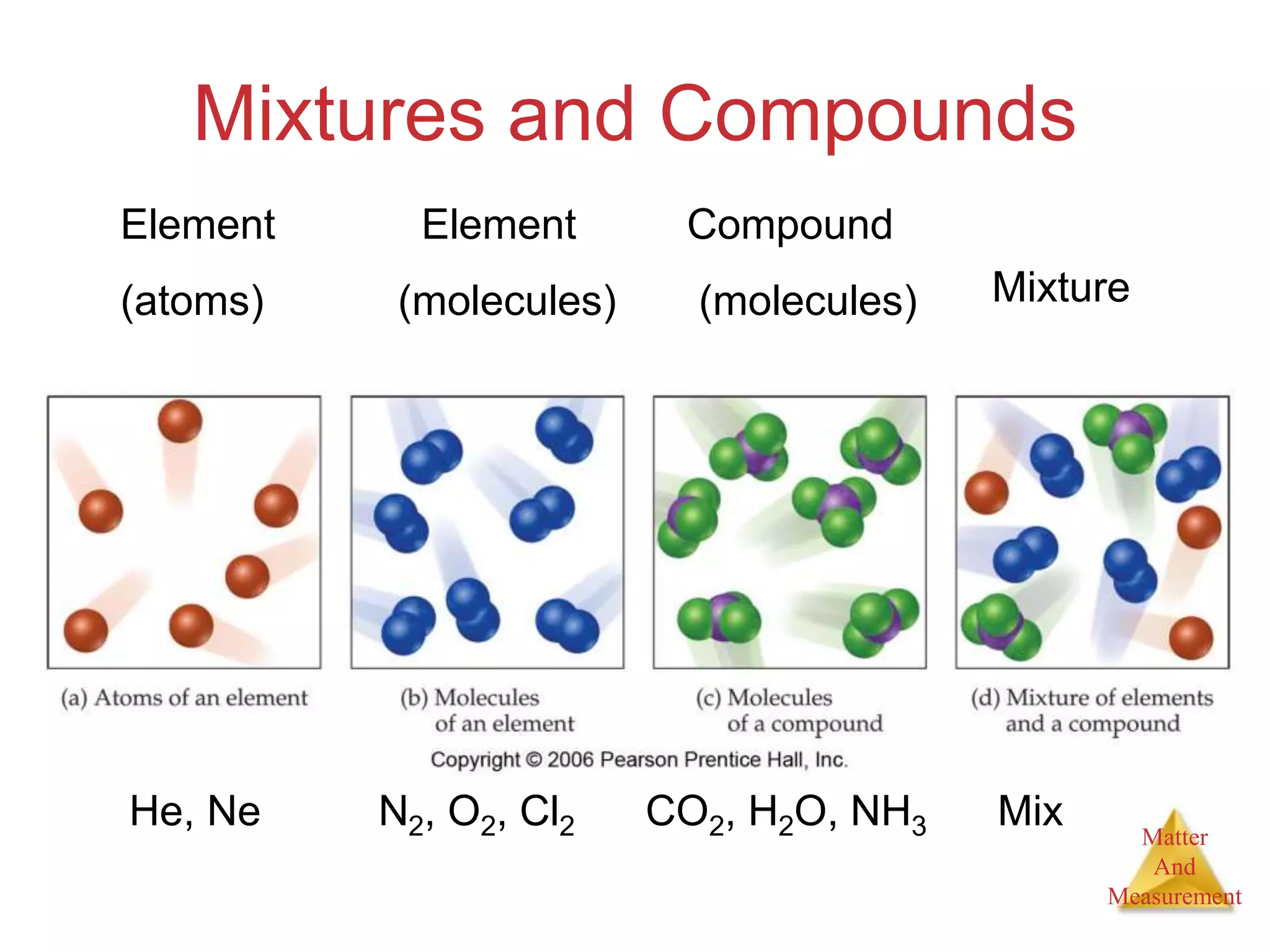
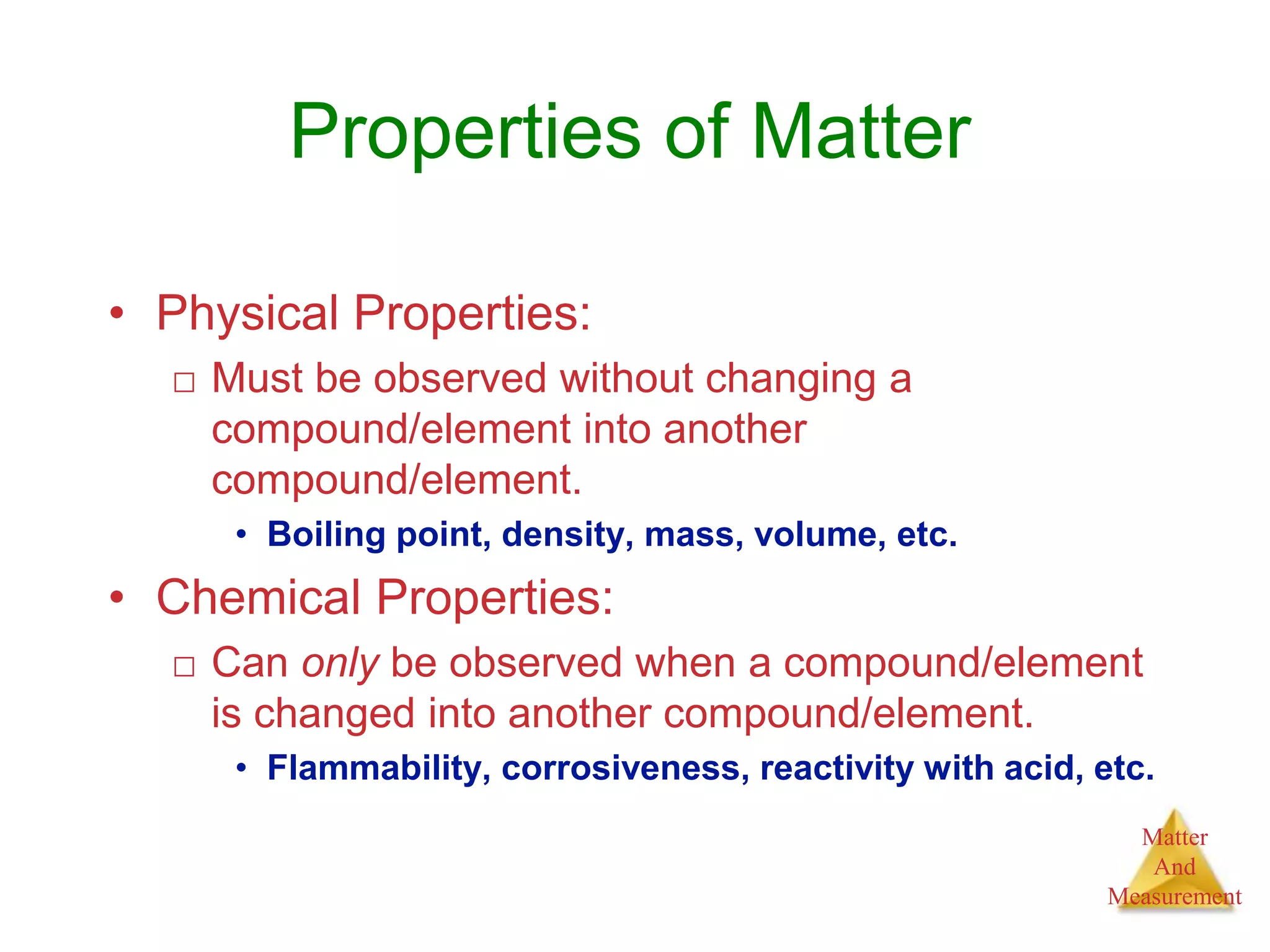
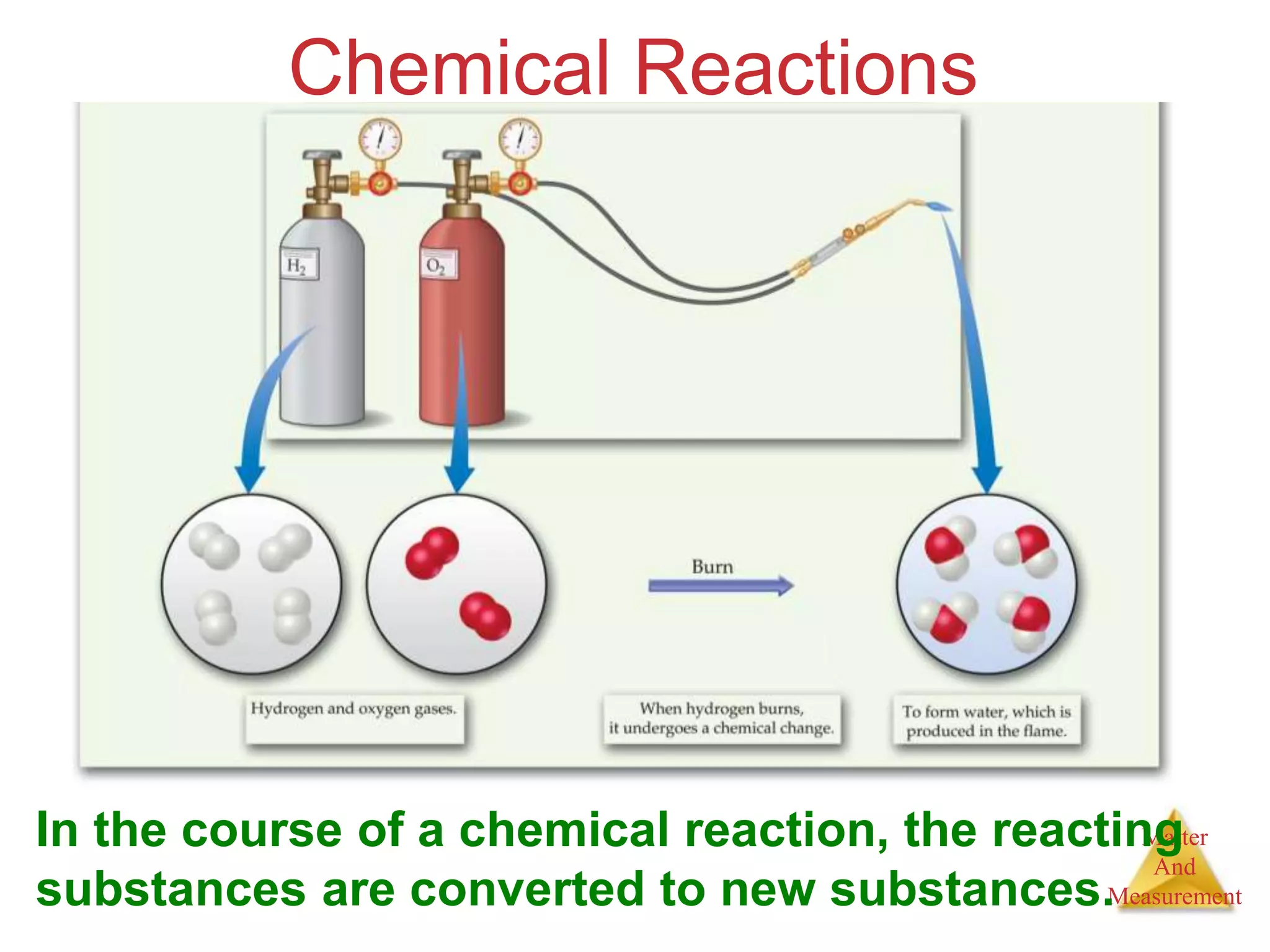
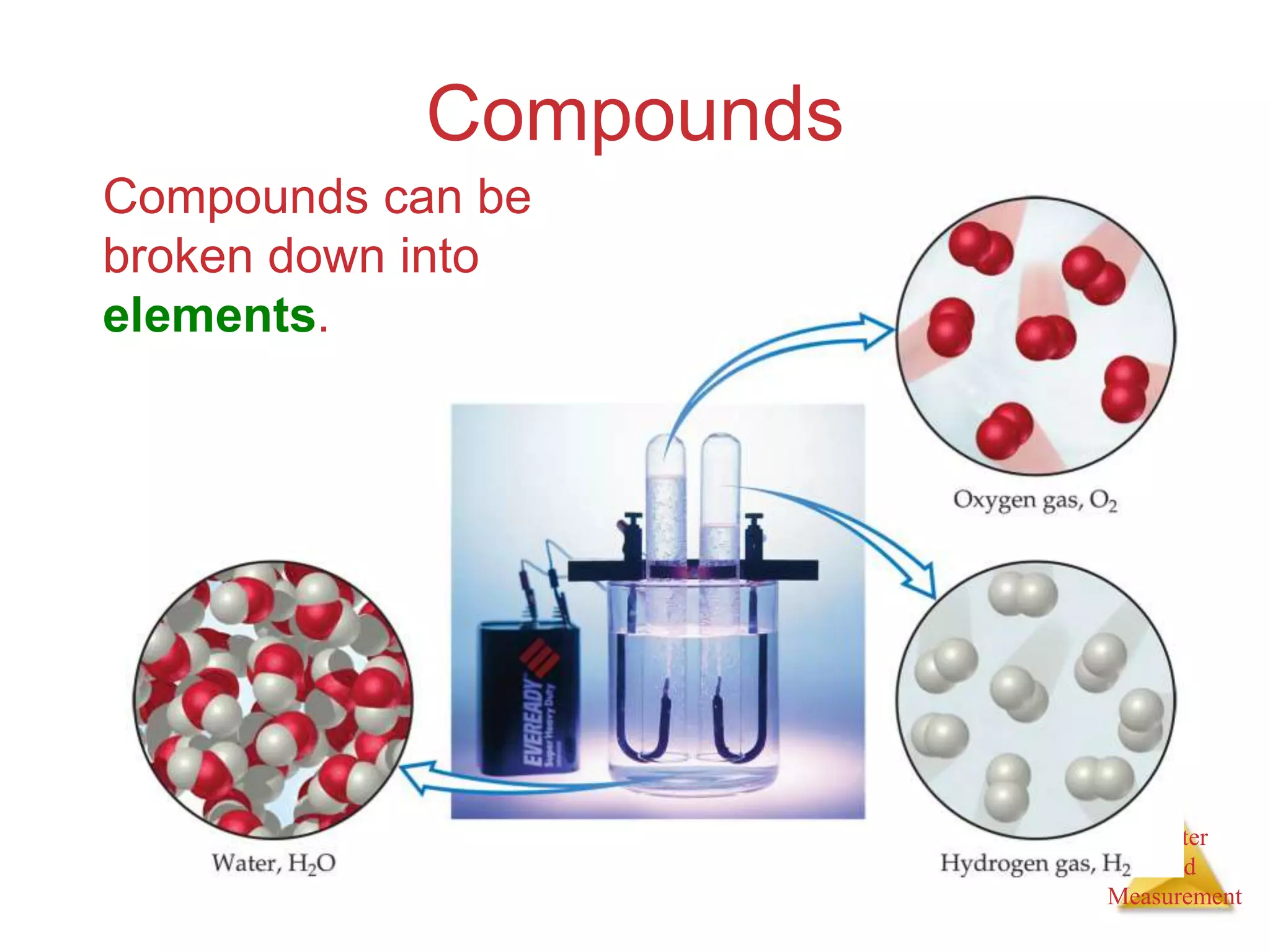
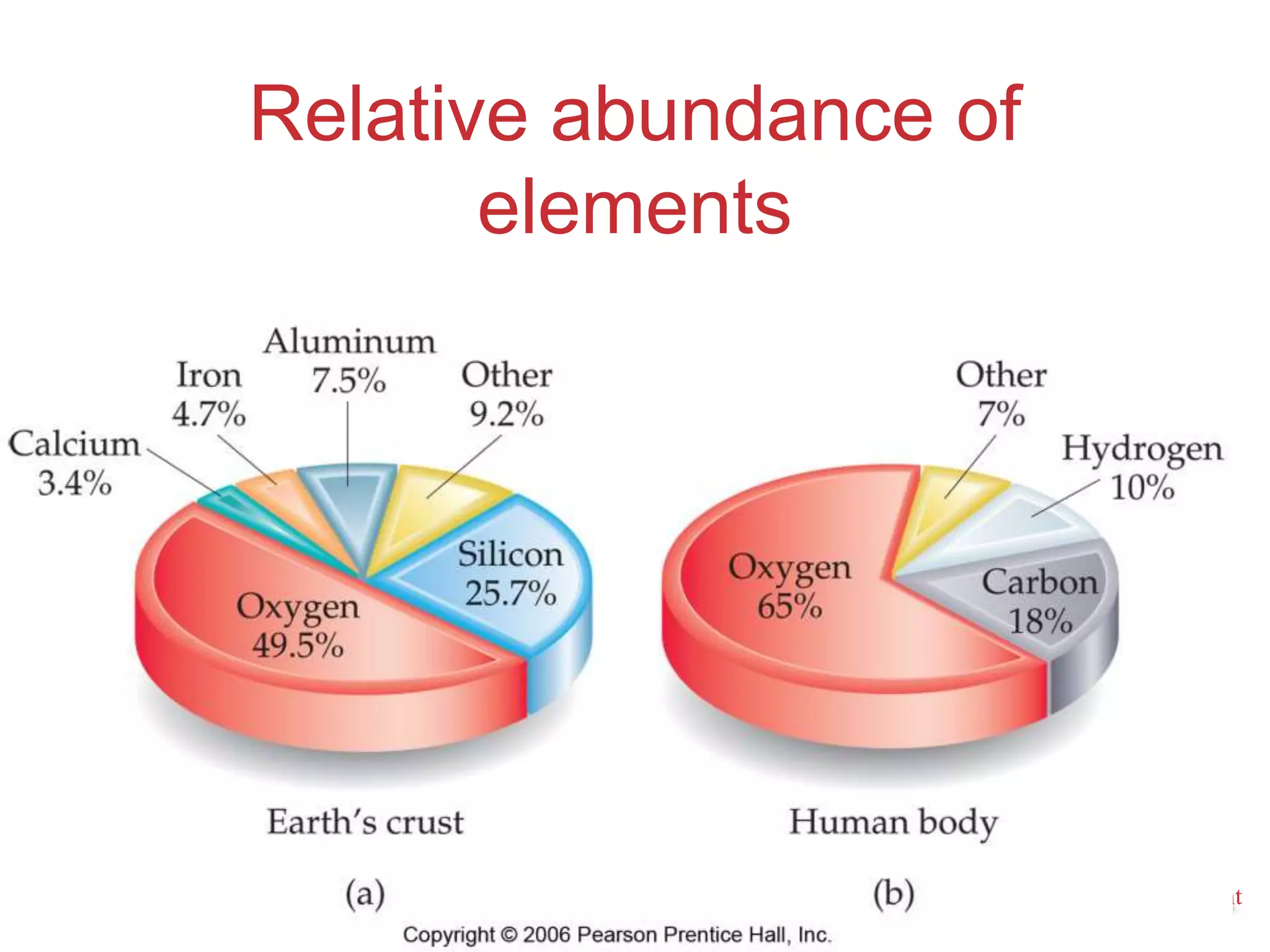
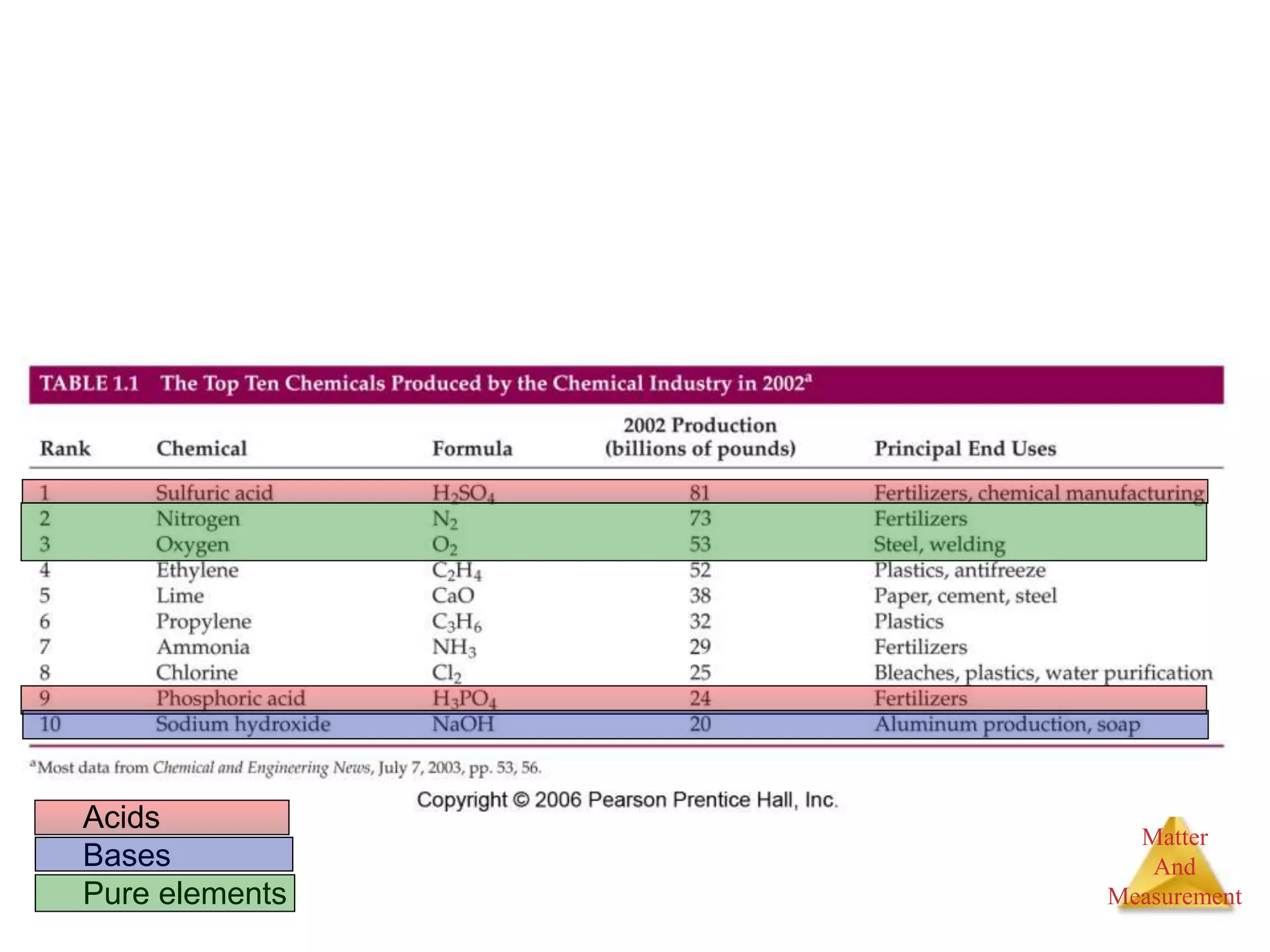
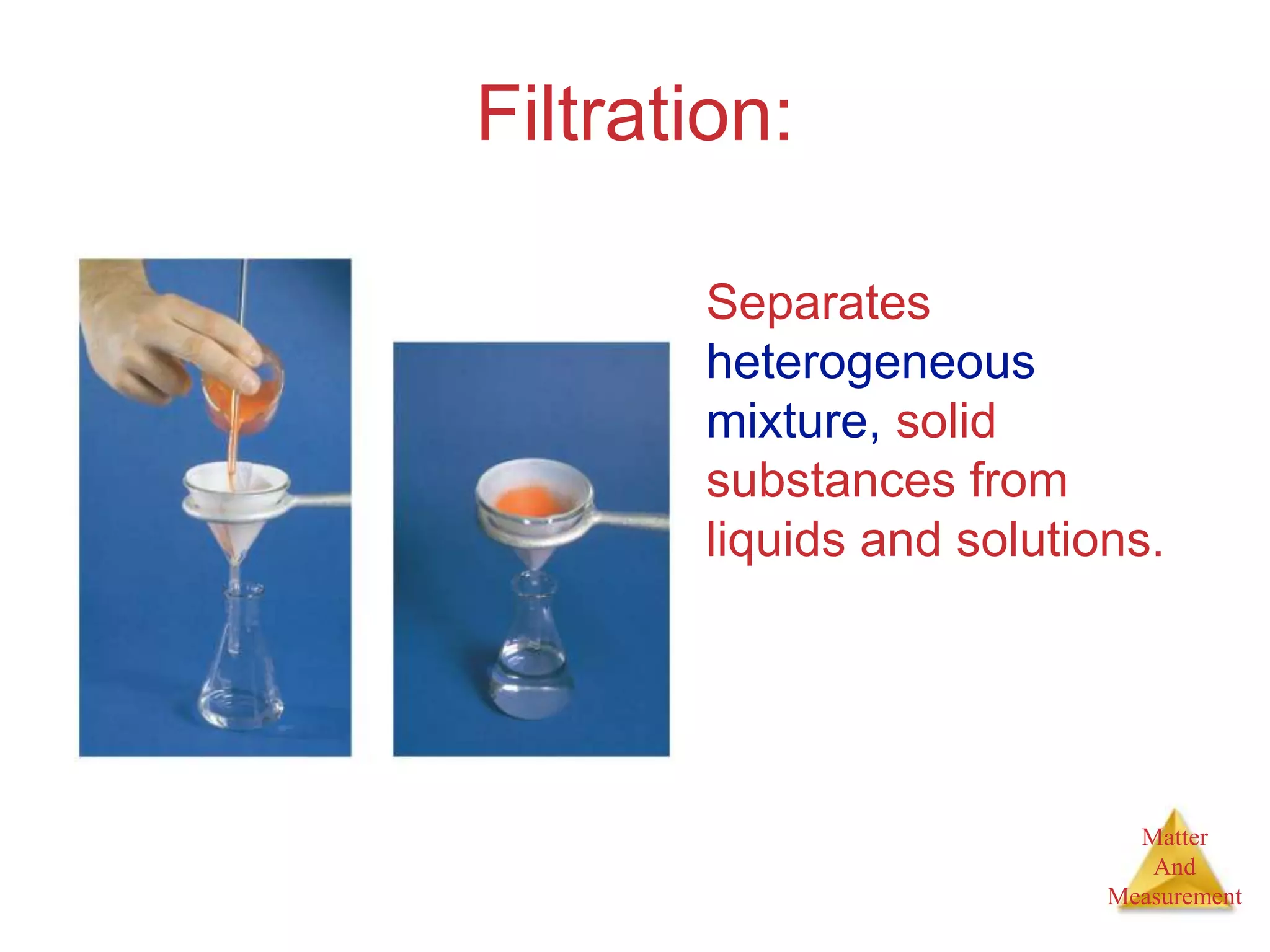
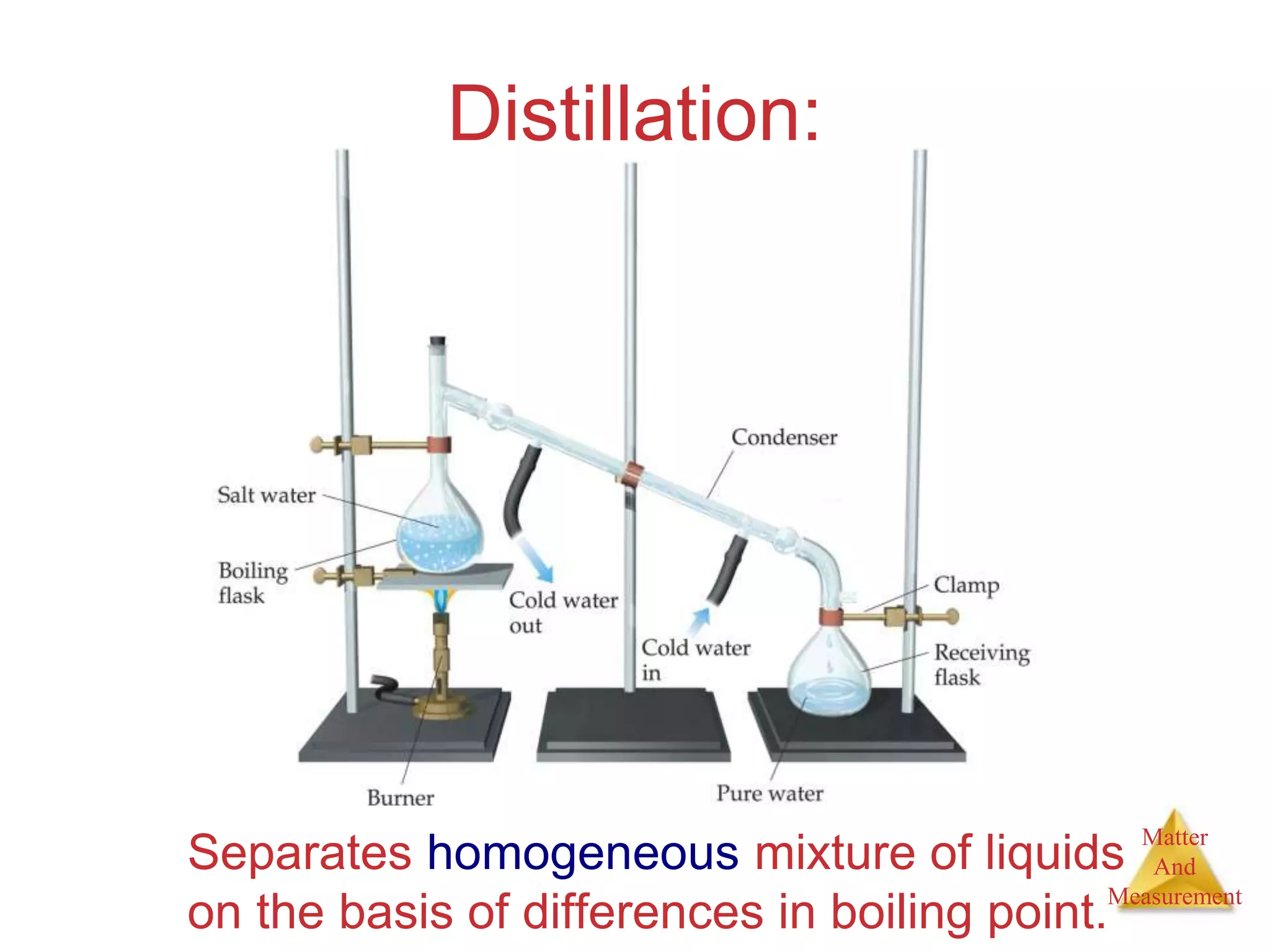
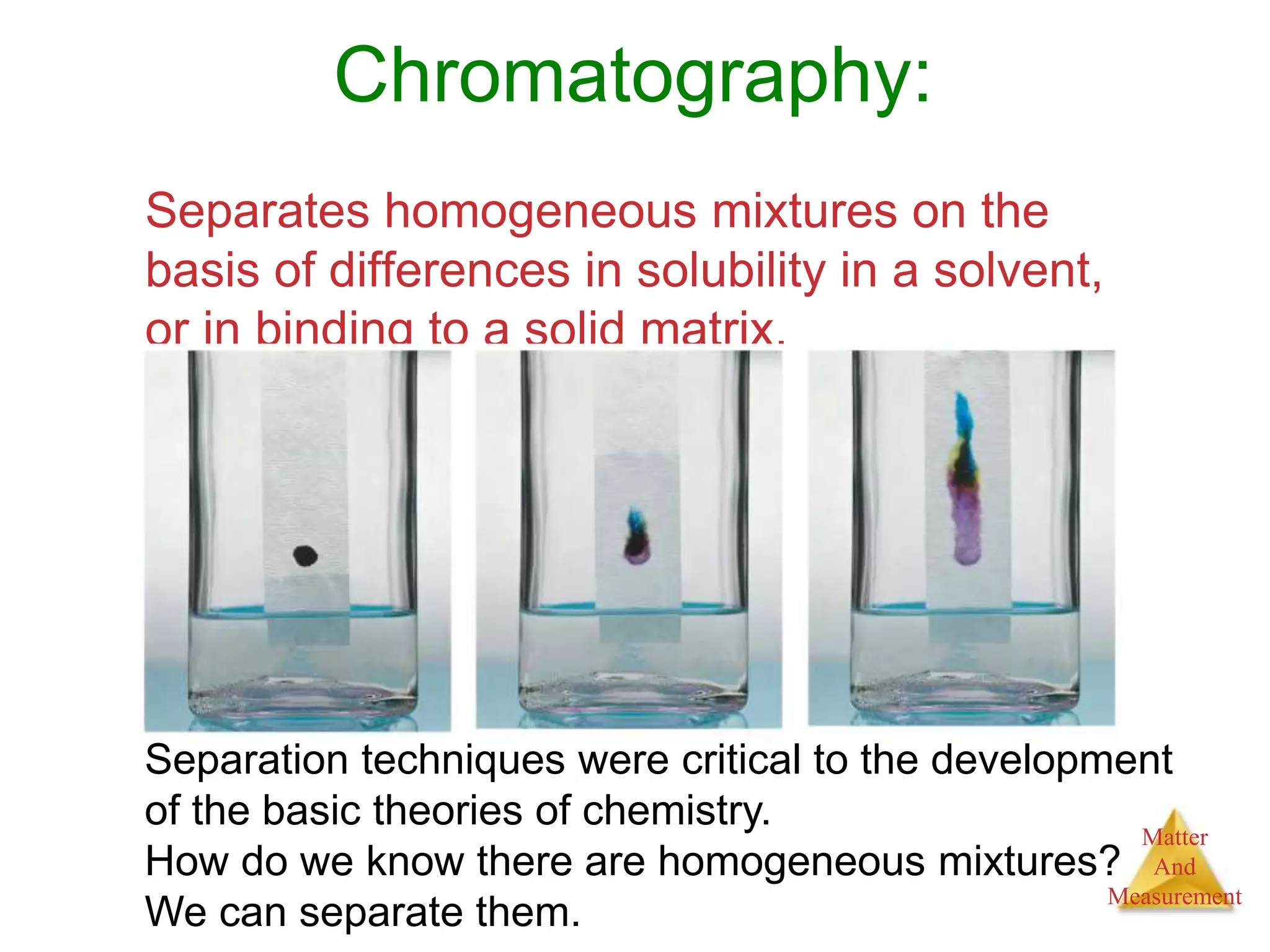
2. Key concepts are defined, such as the three states of matter, differences between elements, compounds and mixtures, physical and chemical properties and changes, and techniques for separating mixtures.
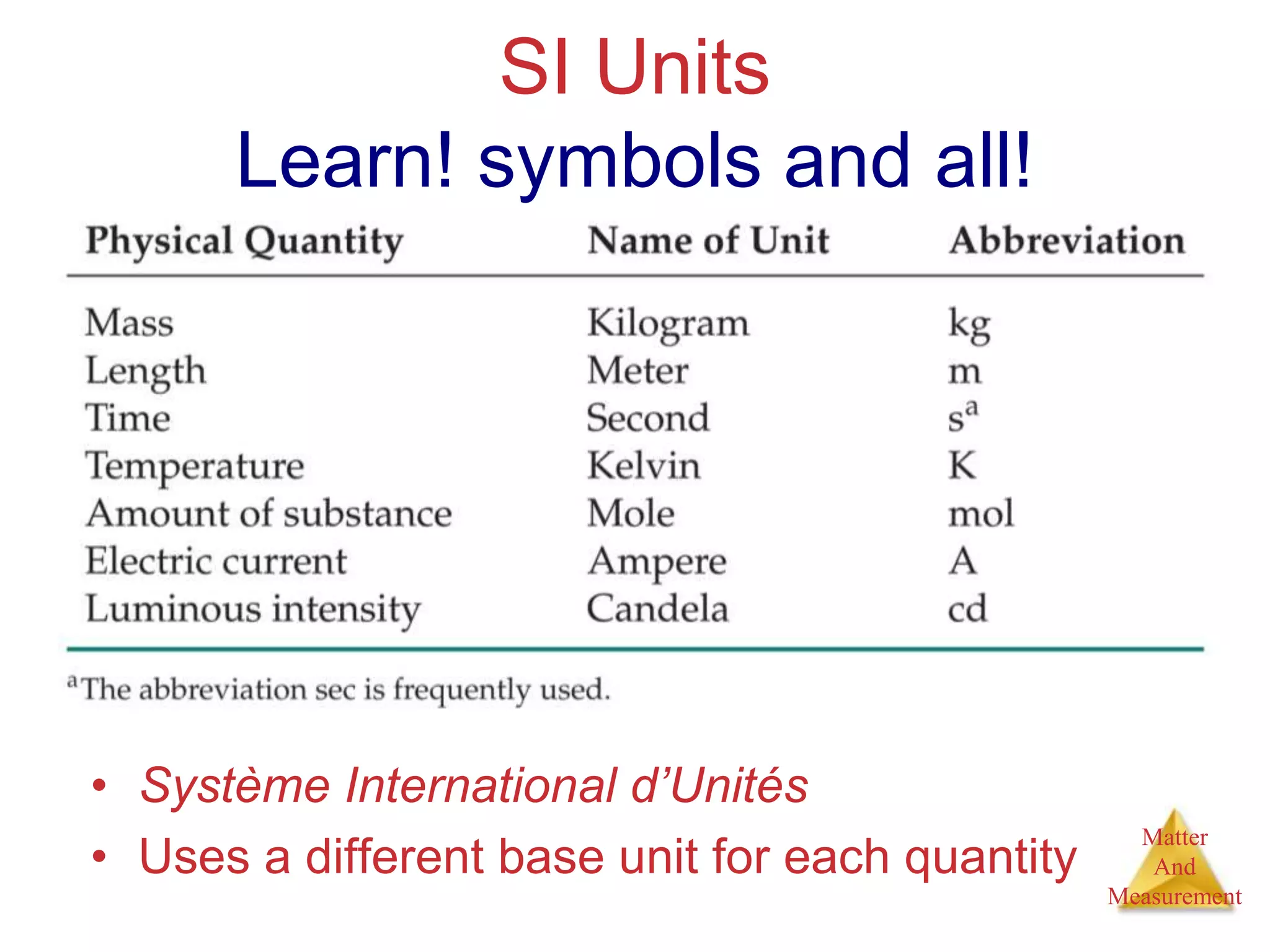
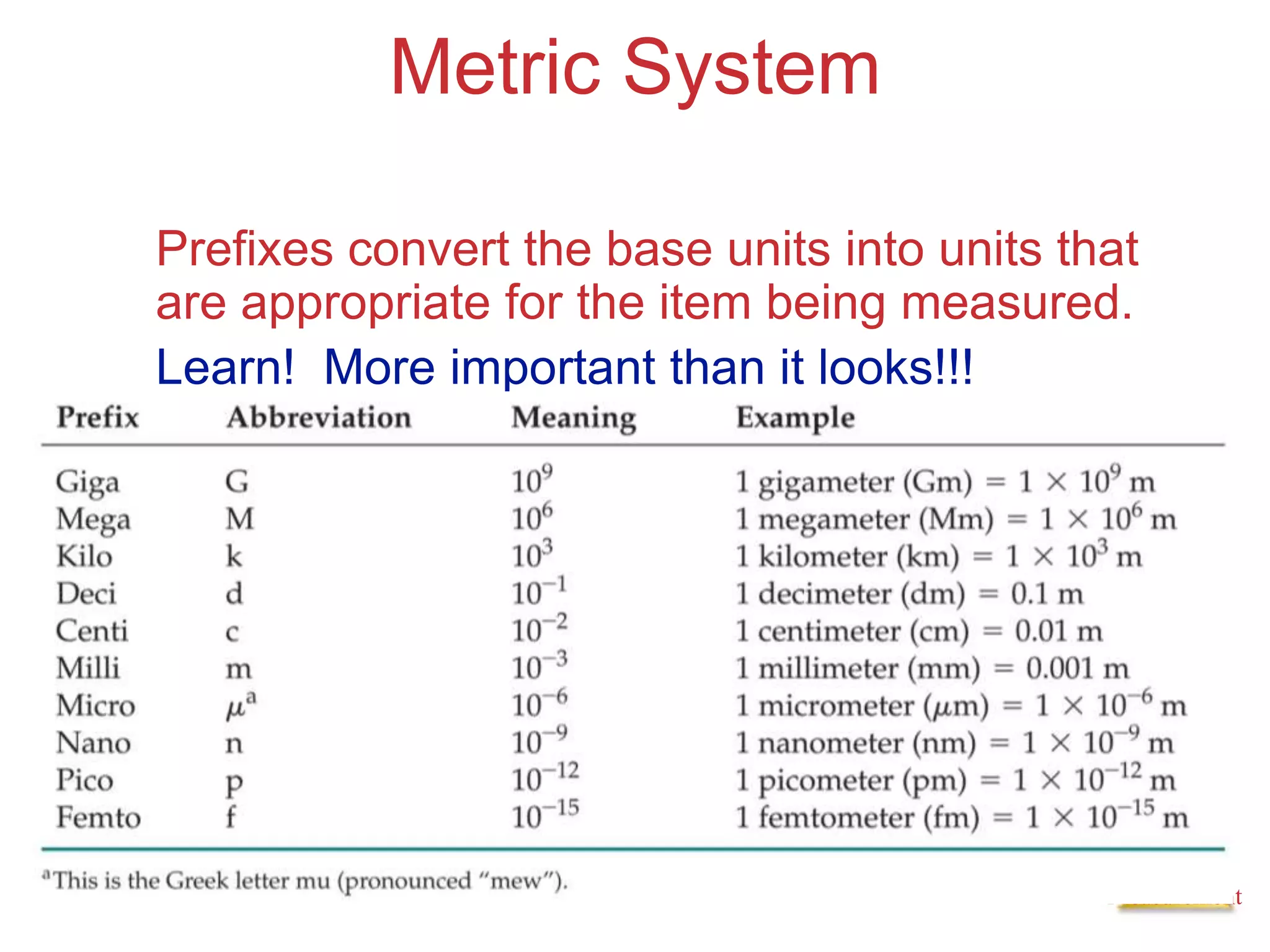
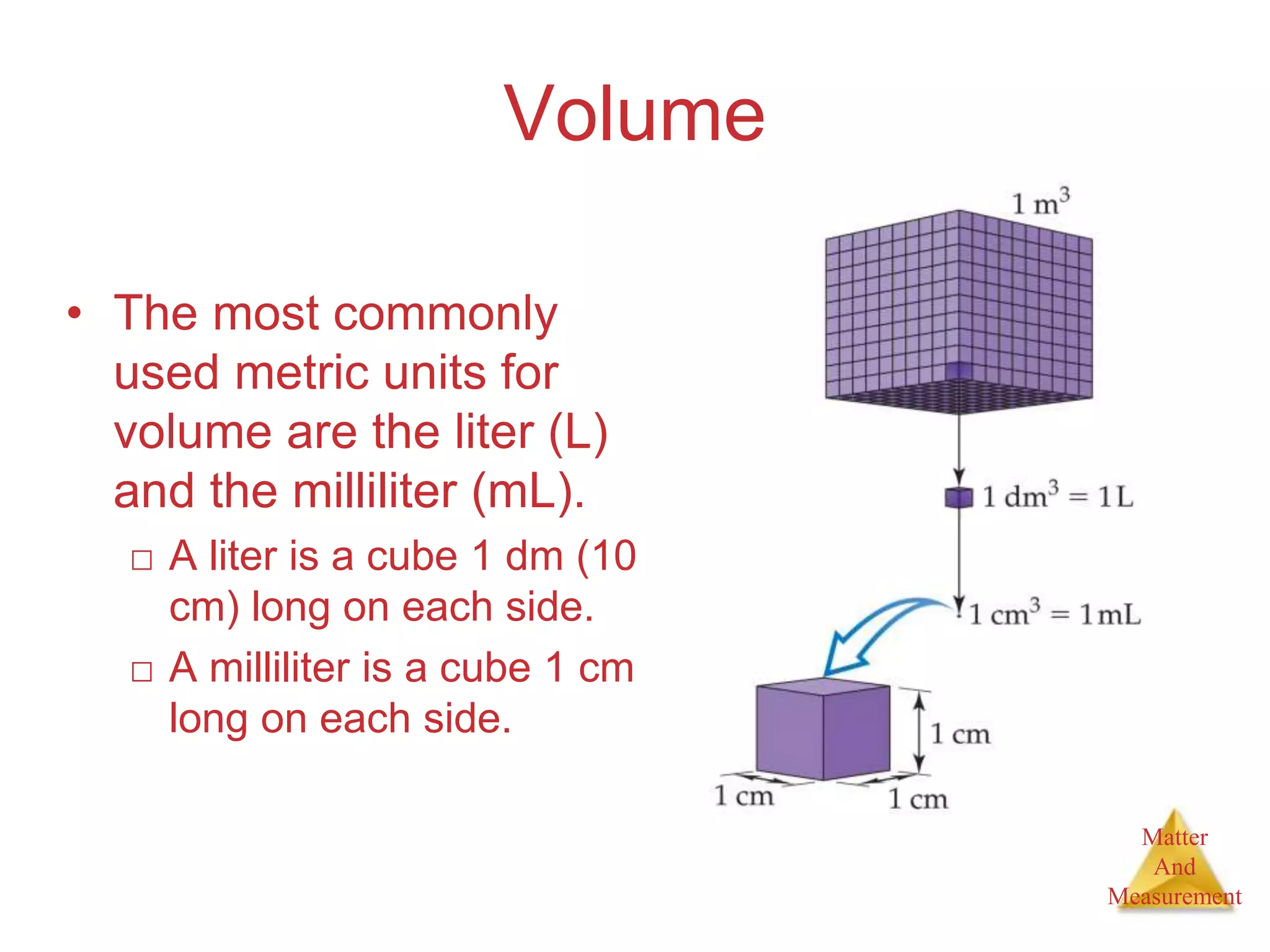
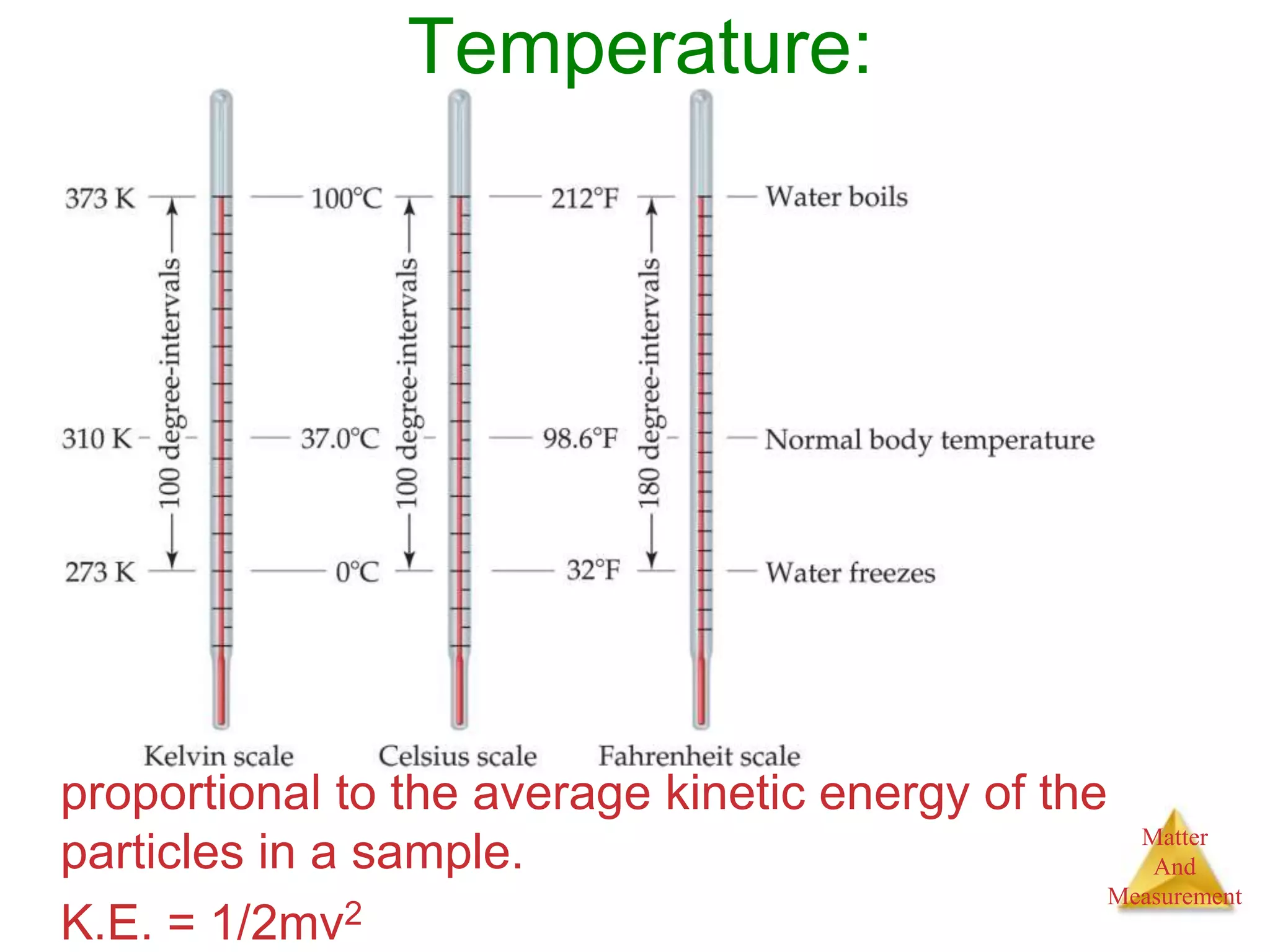
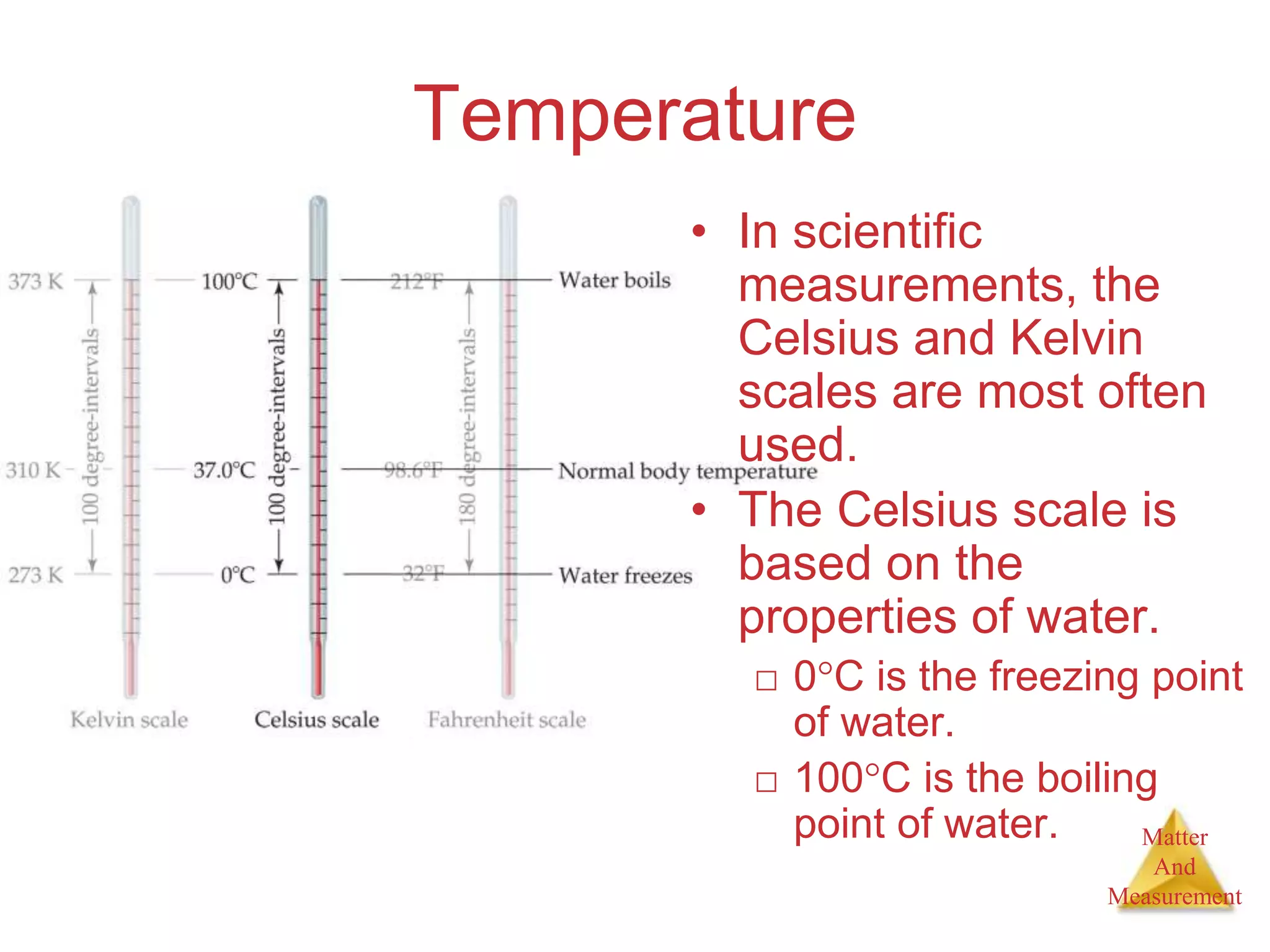
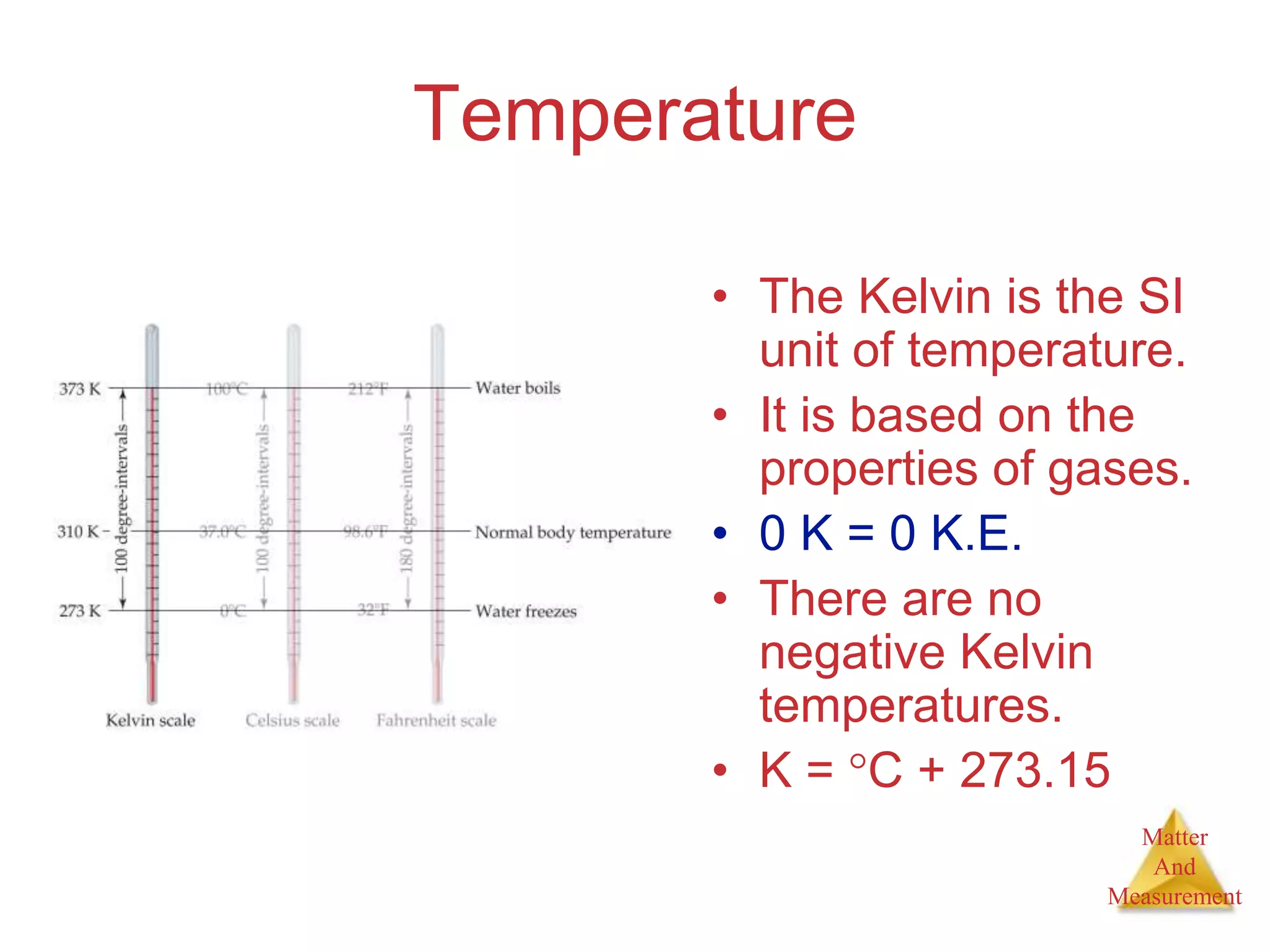
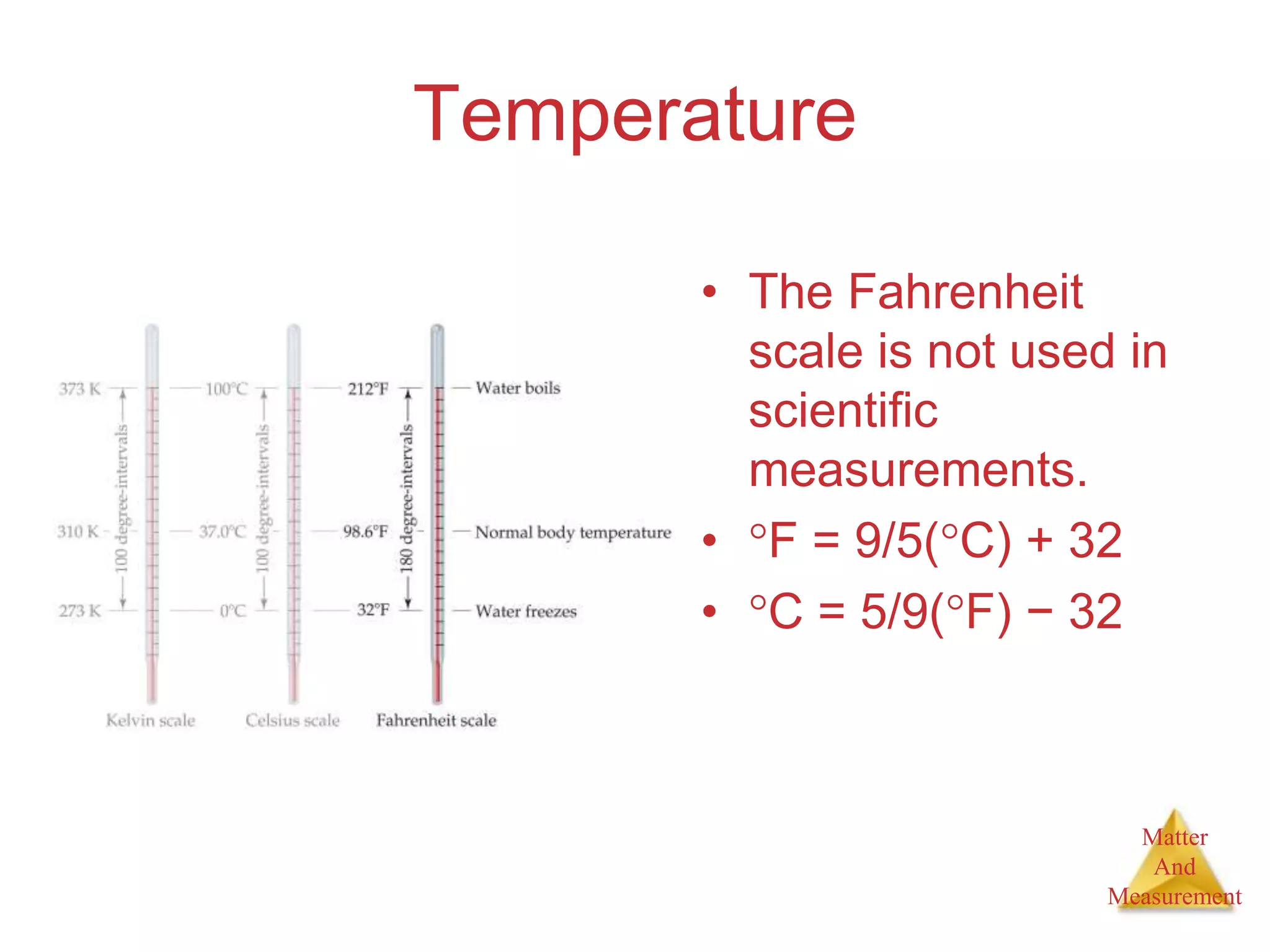
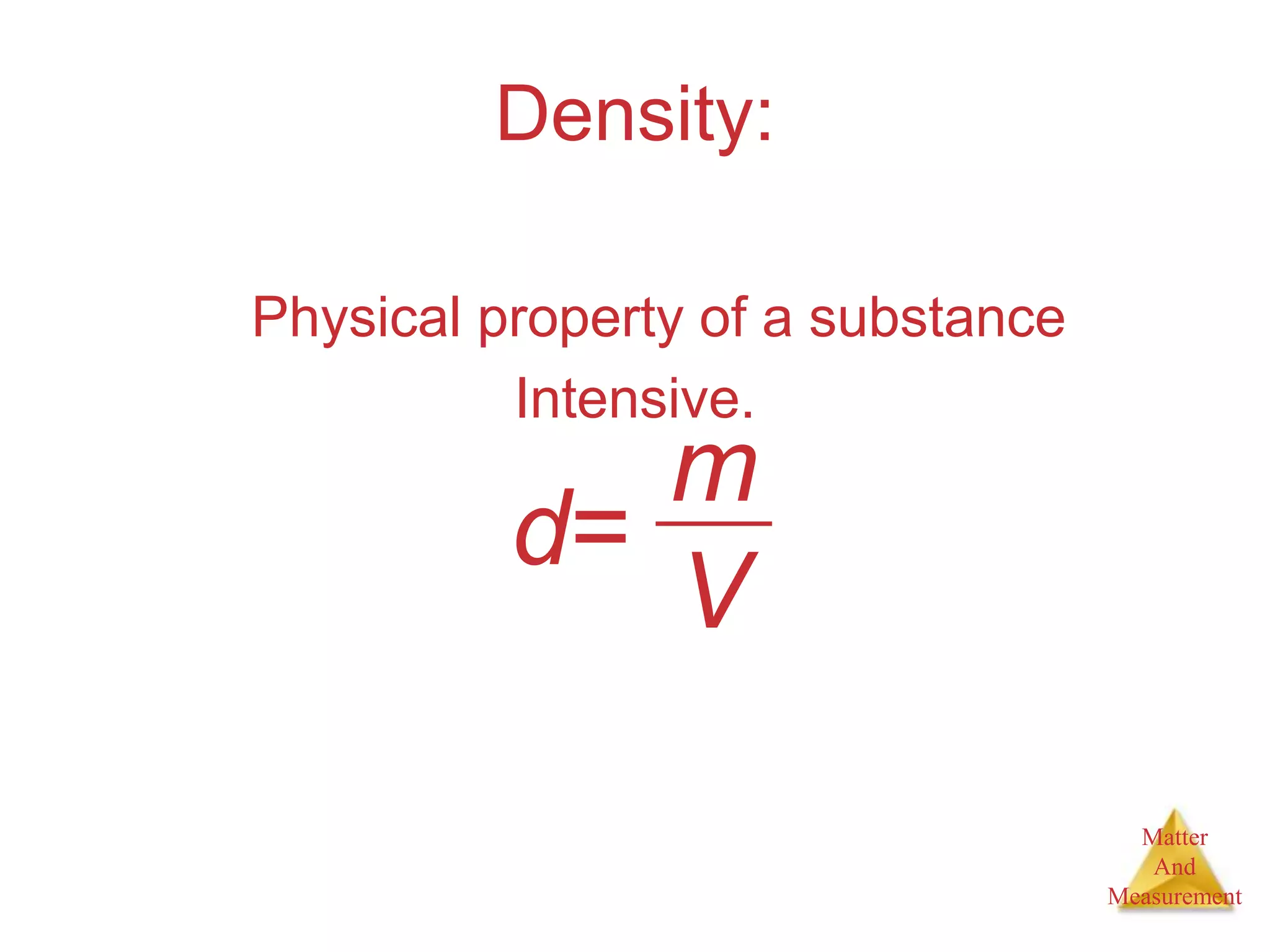
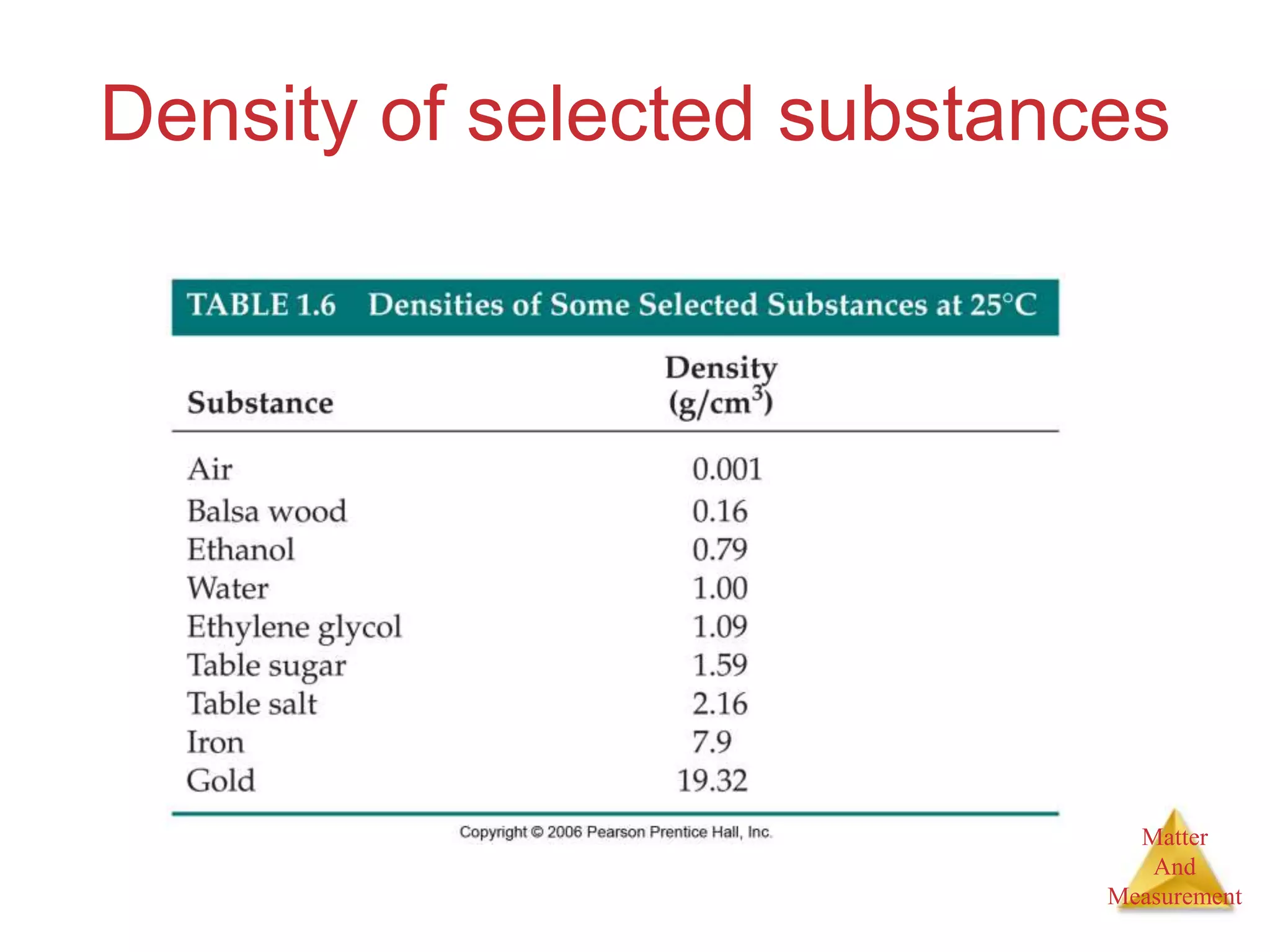
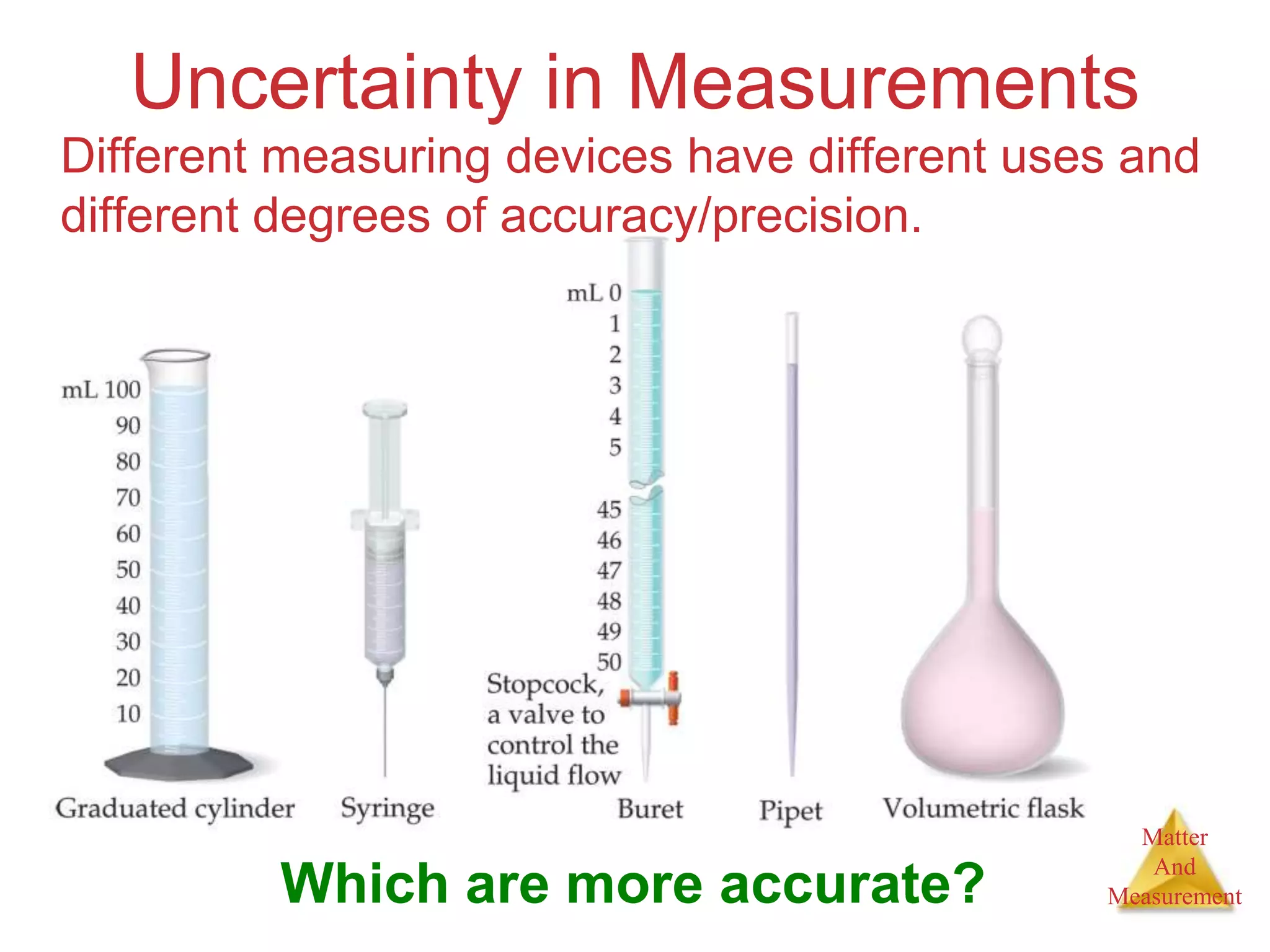
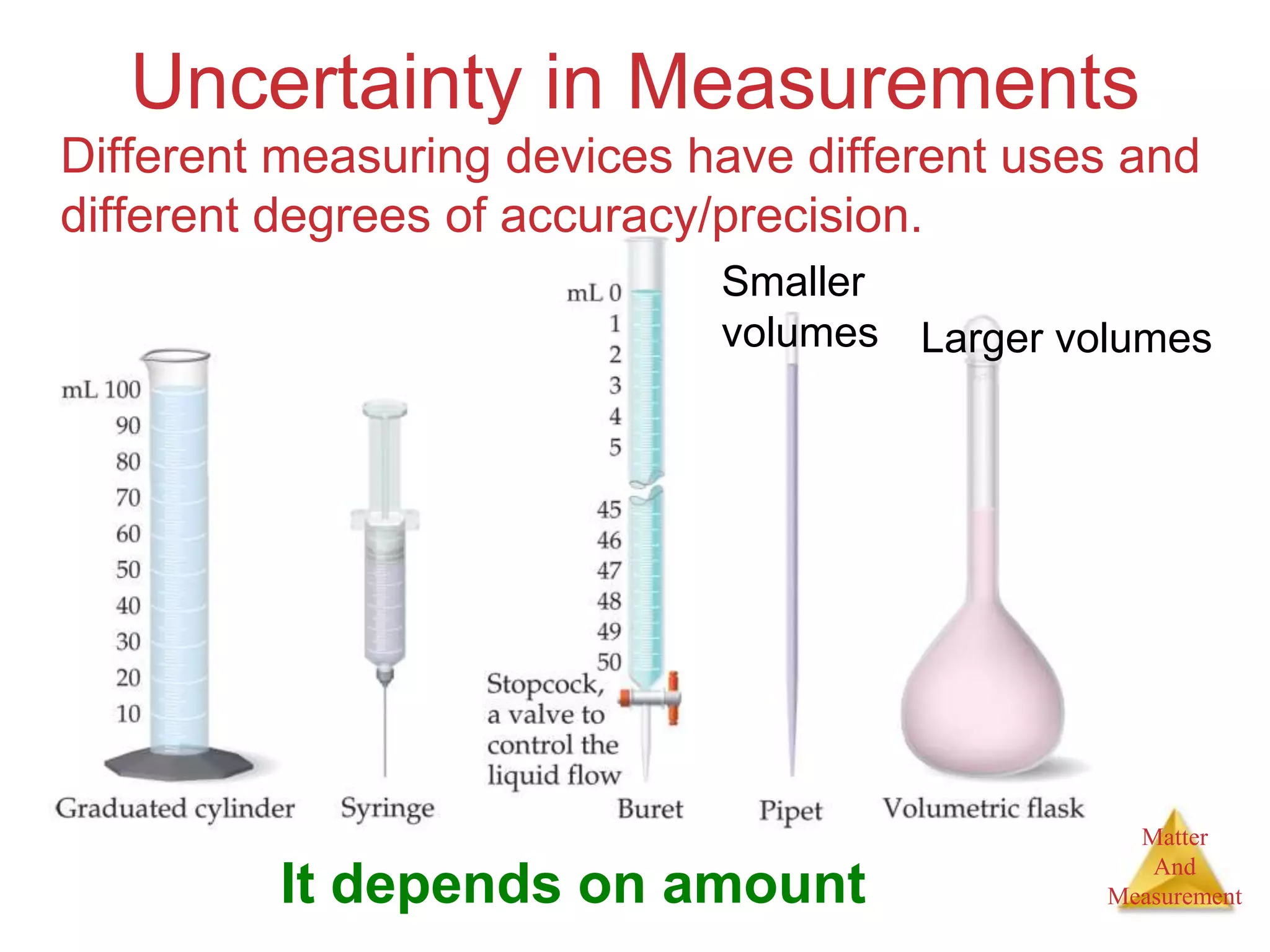
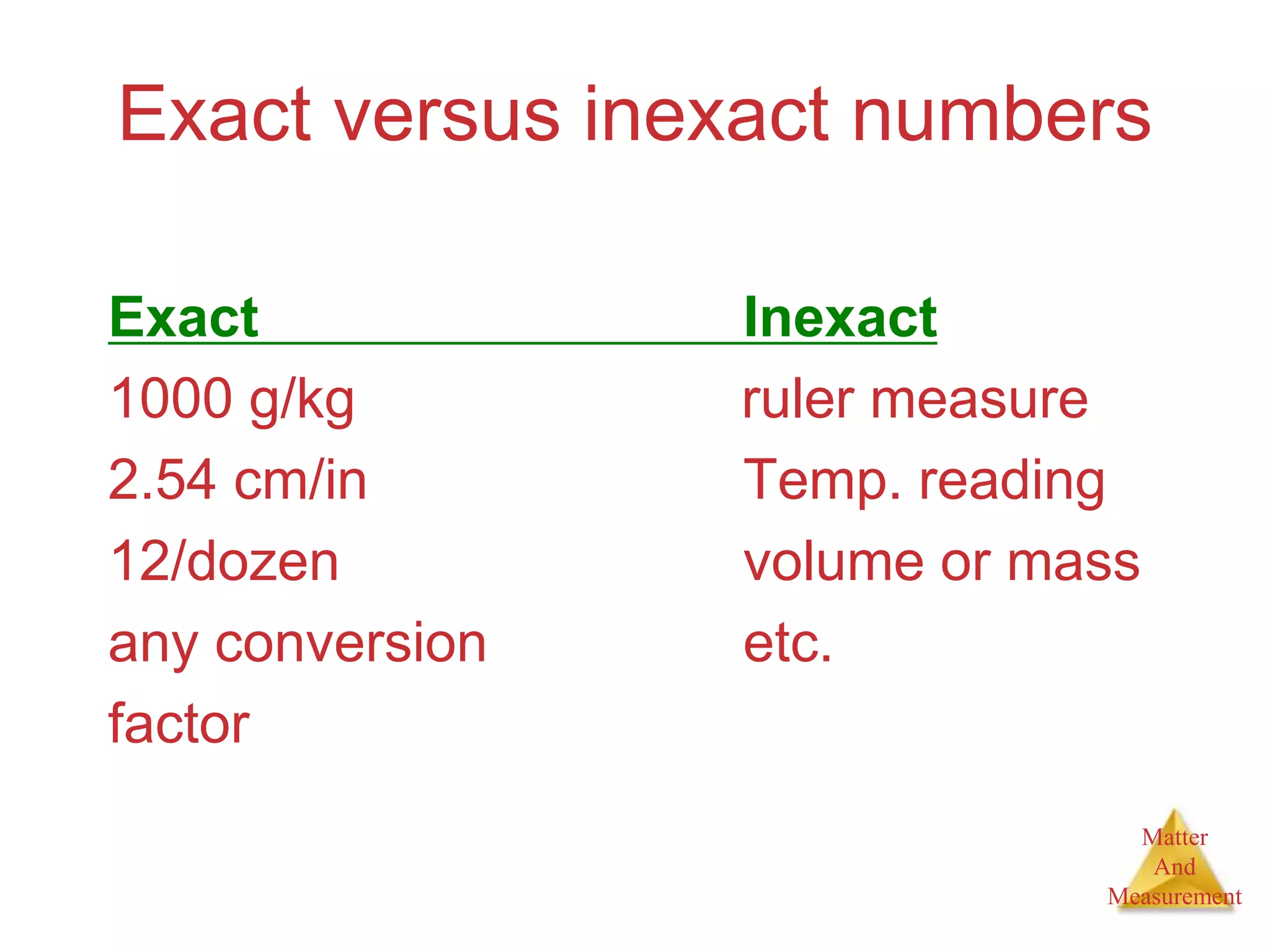
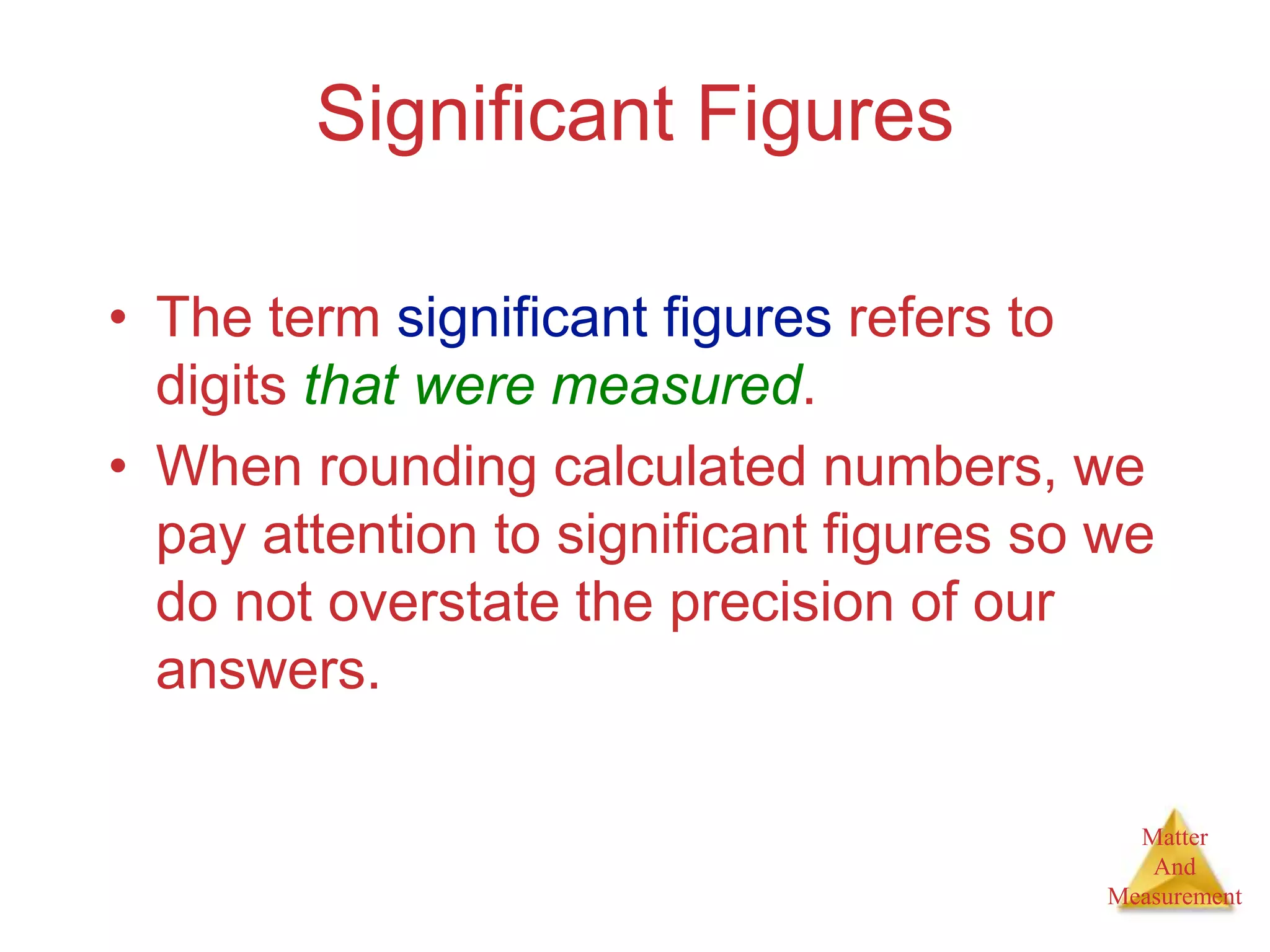
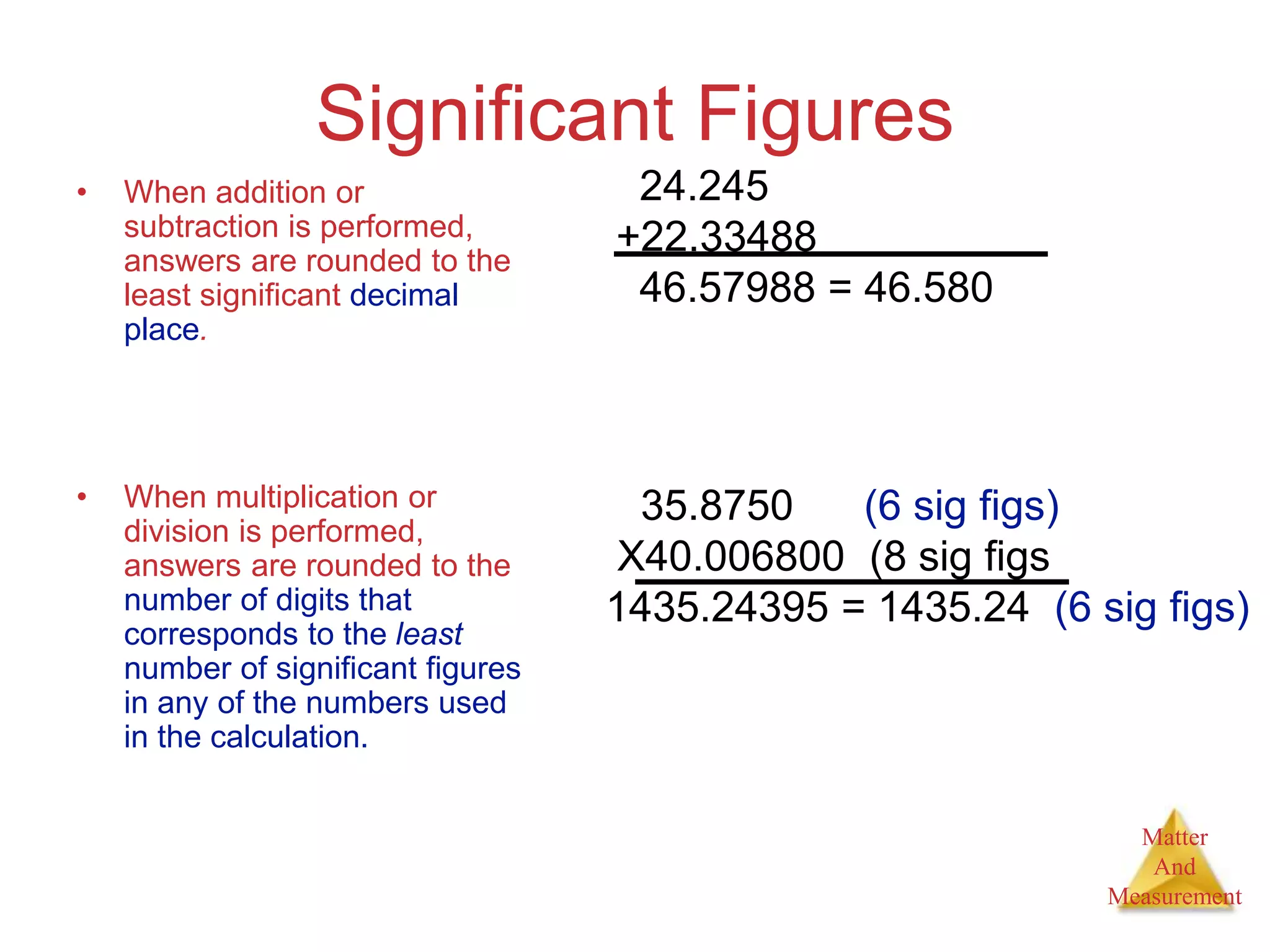
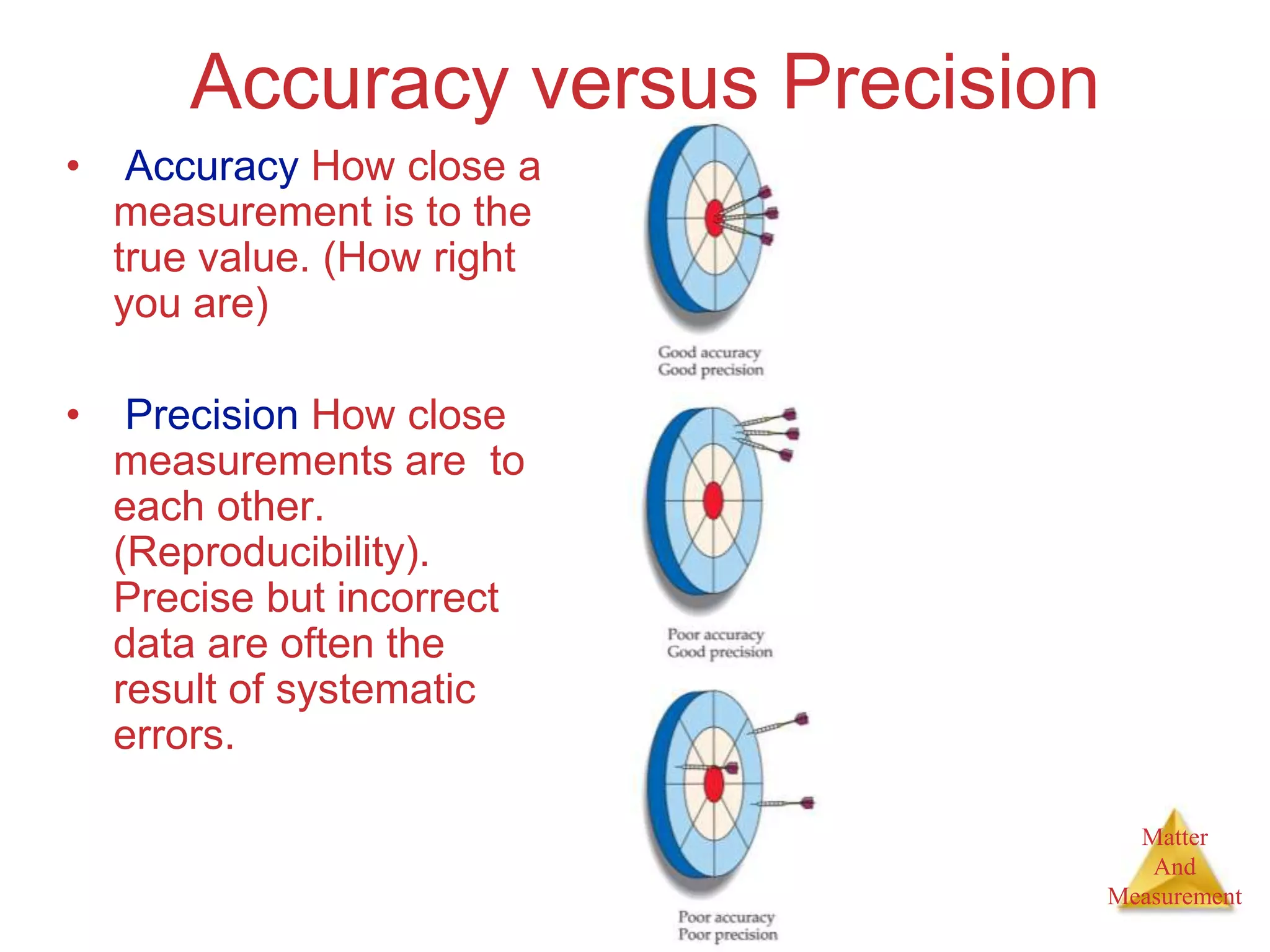
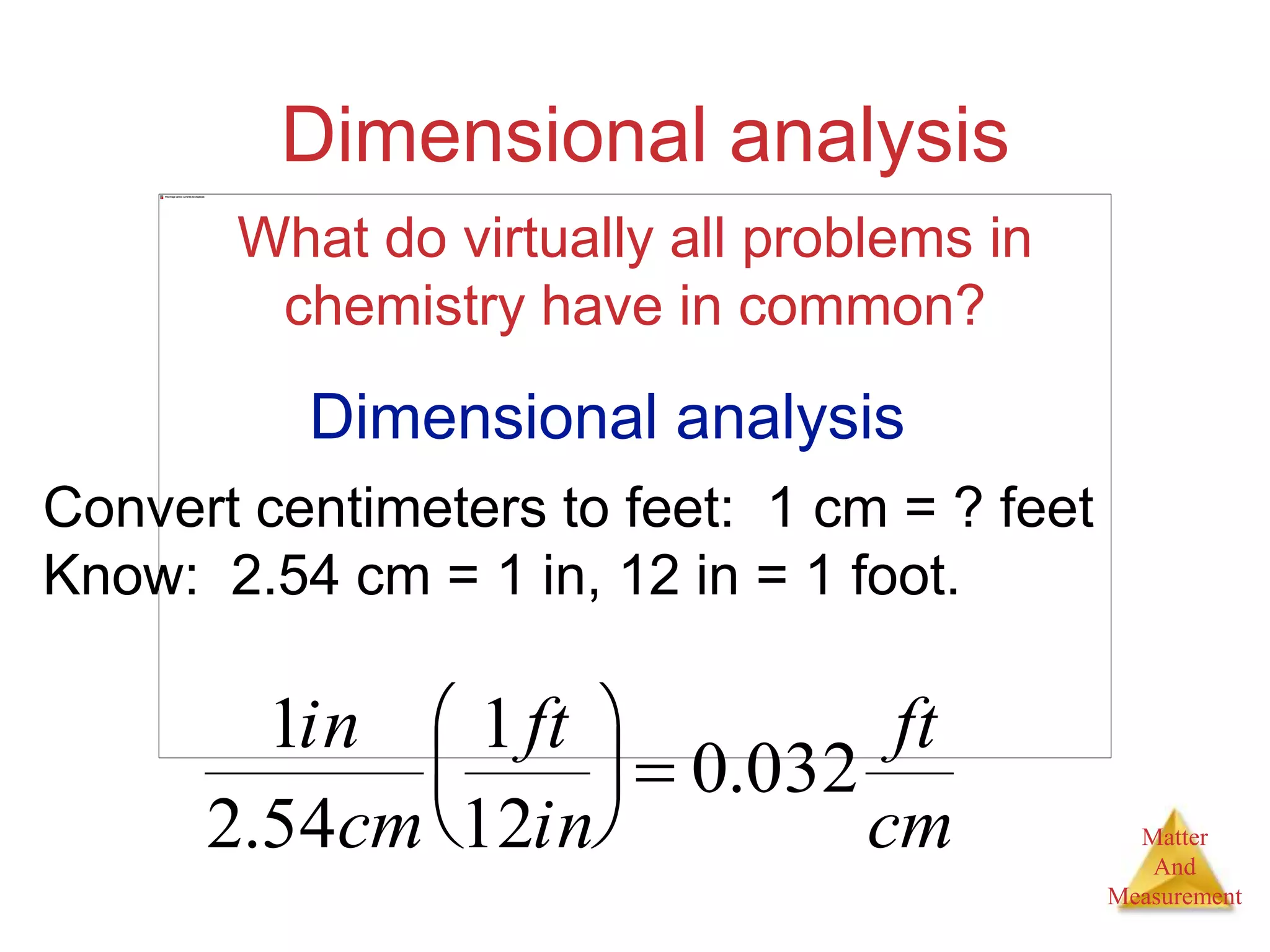
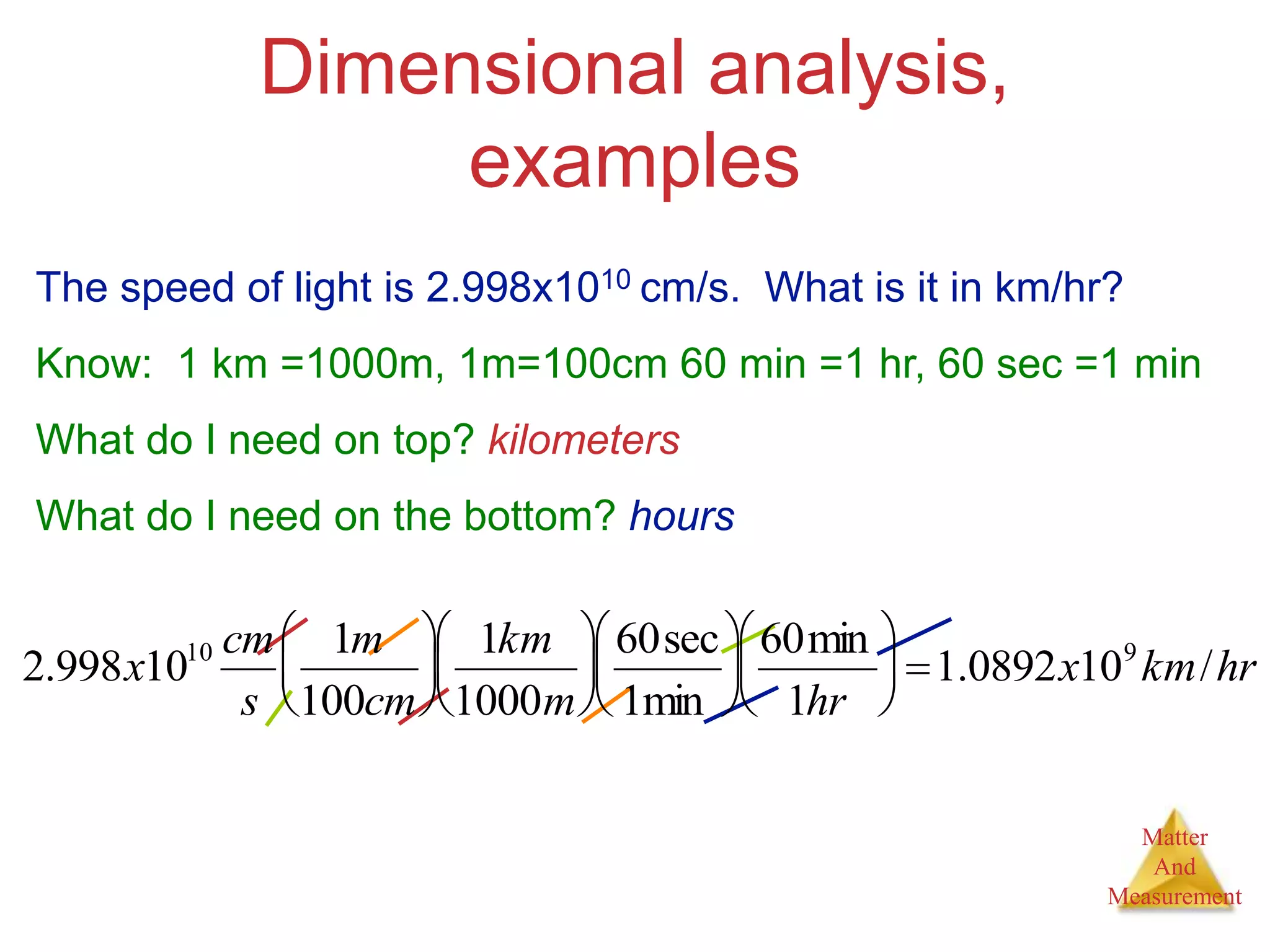
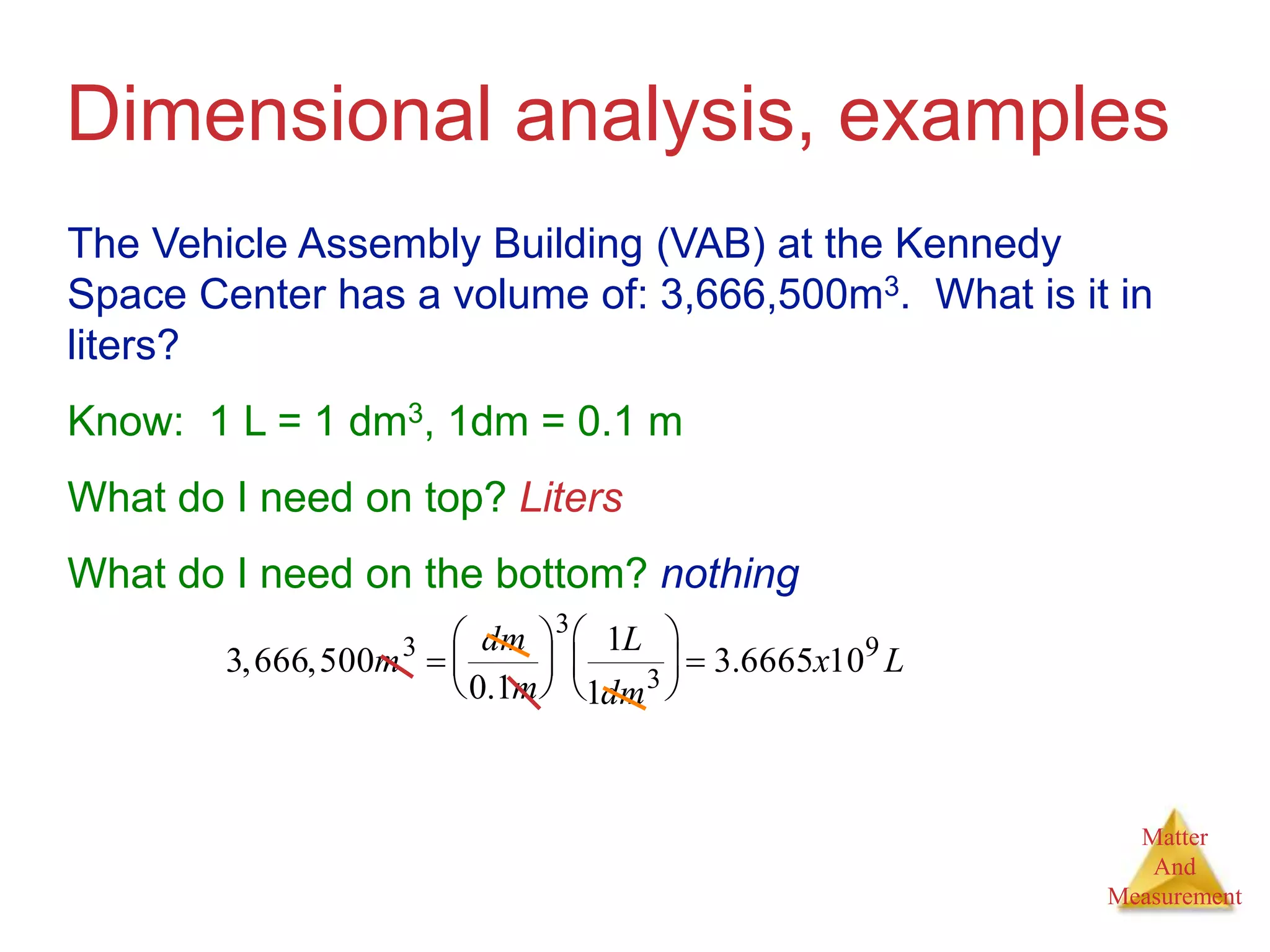
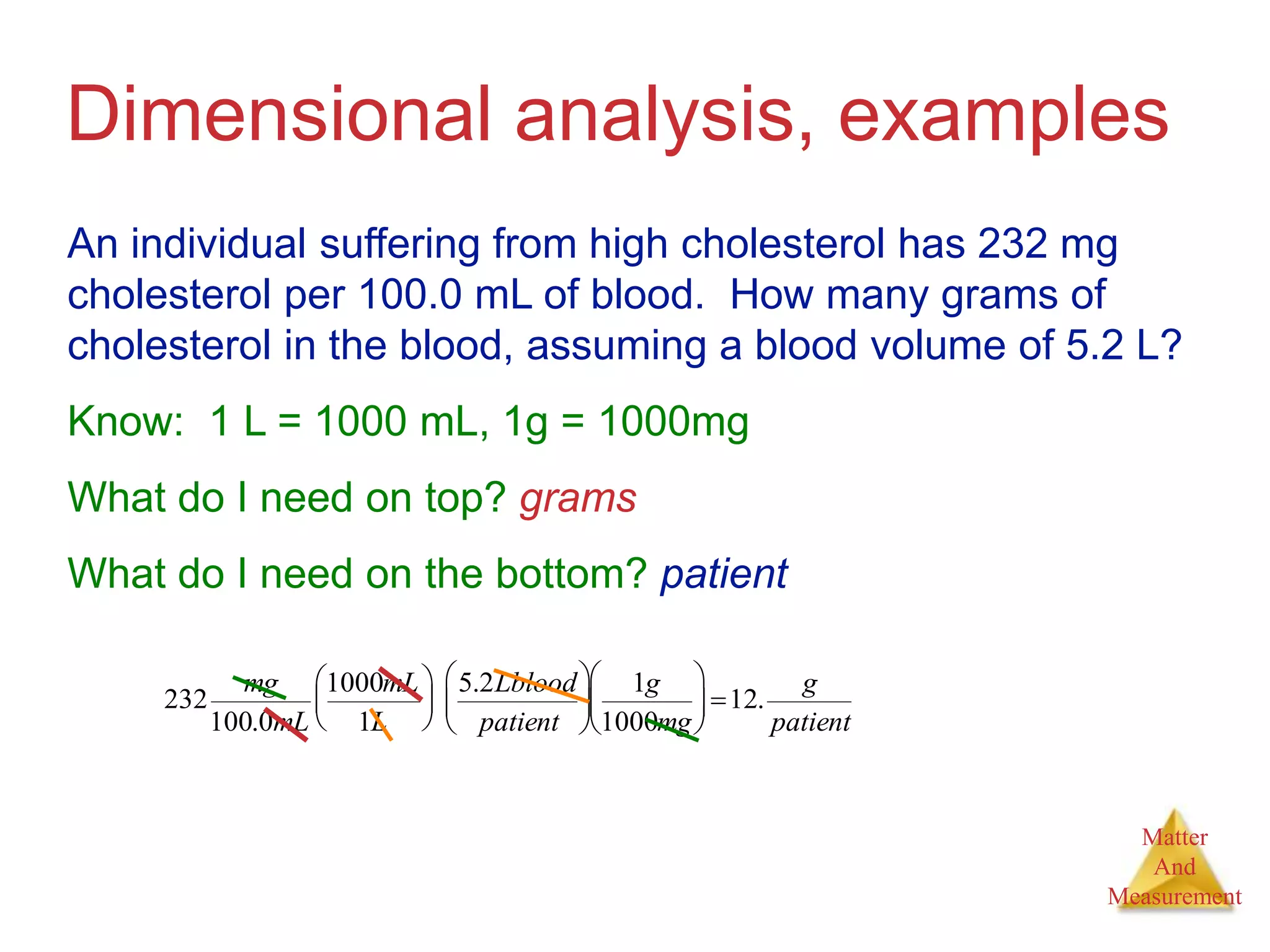
3. Common units used to measure properties important in chemistry are described, including the metric system, units for volume, temperature, density and the concepts of accuracy, precision, and significant figures in measurements.