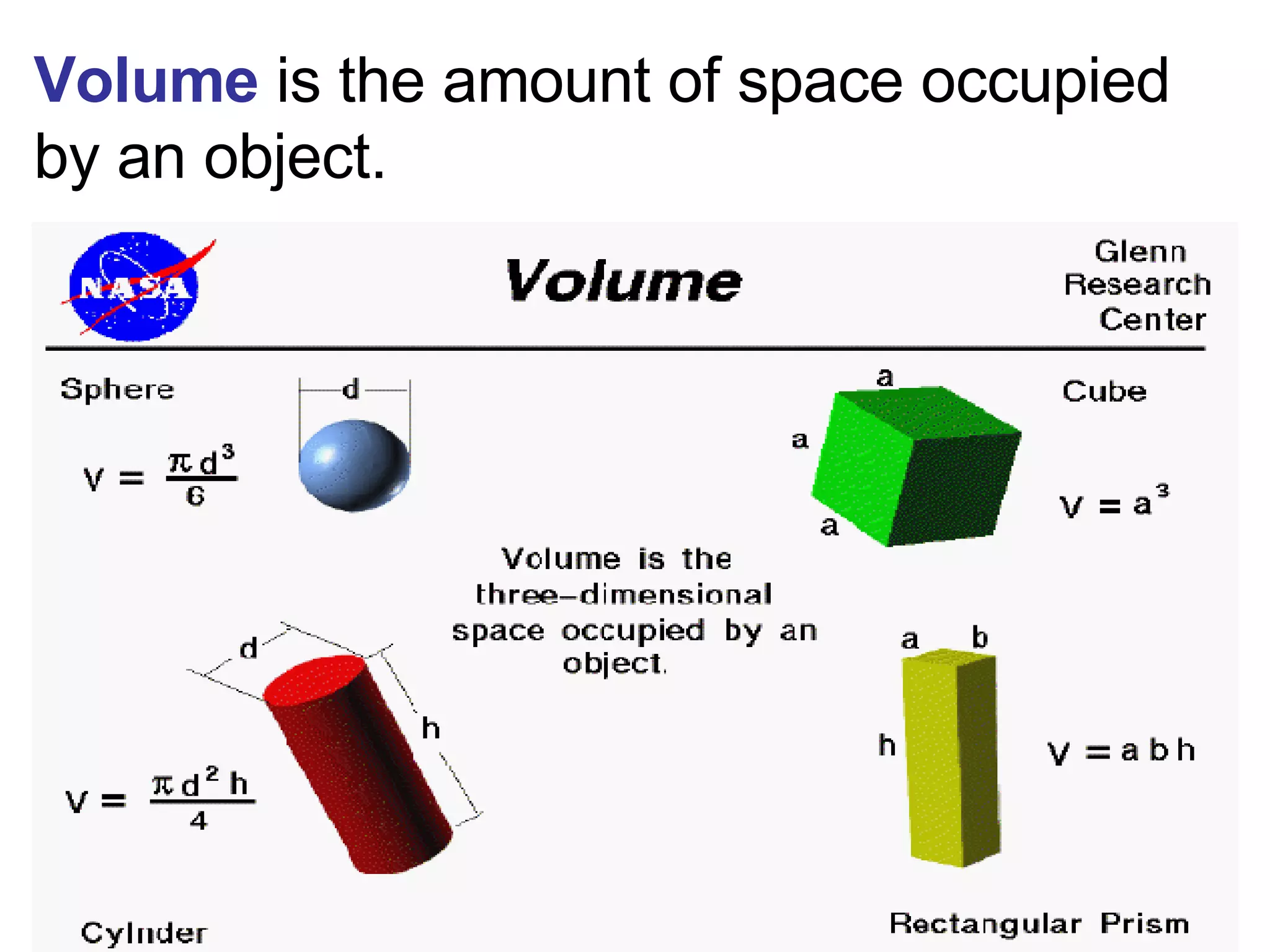
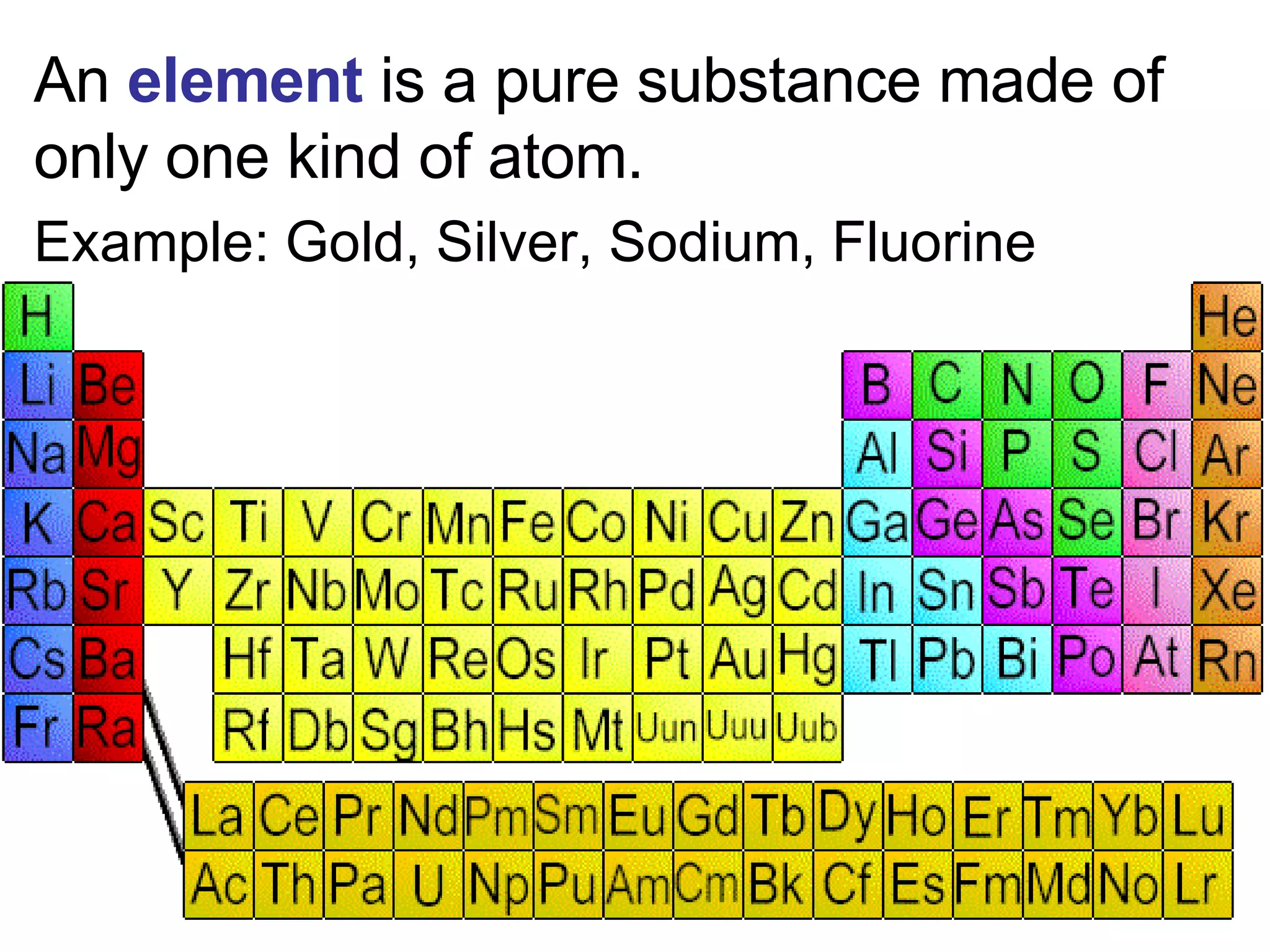
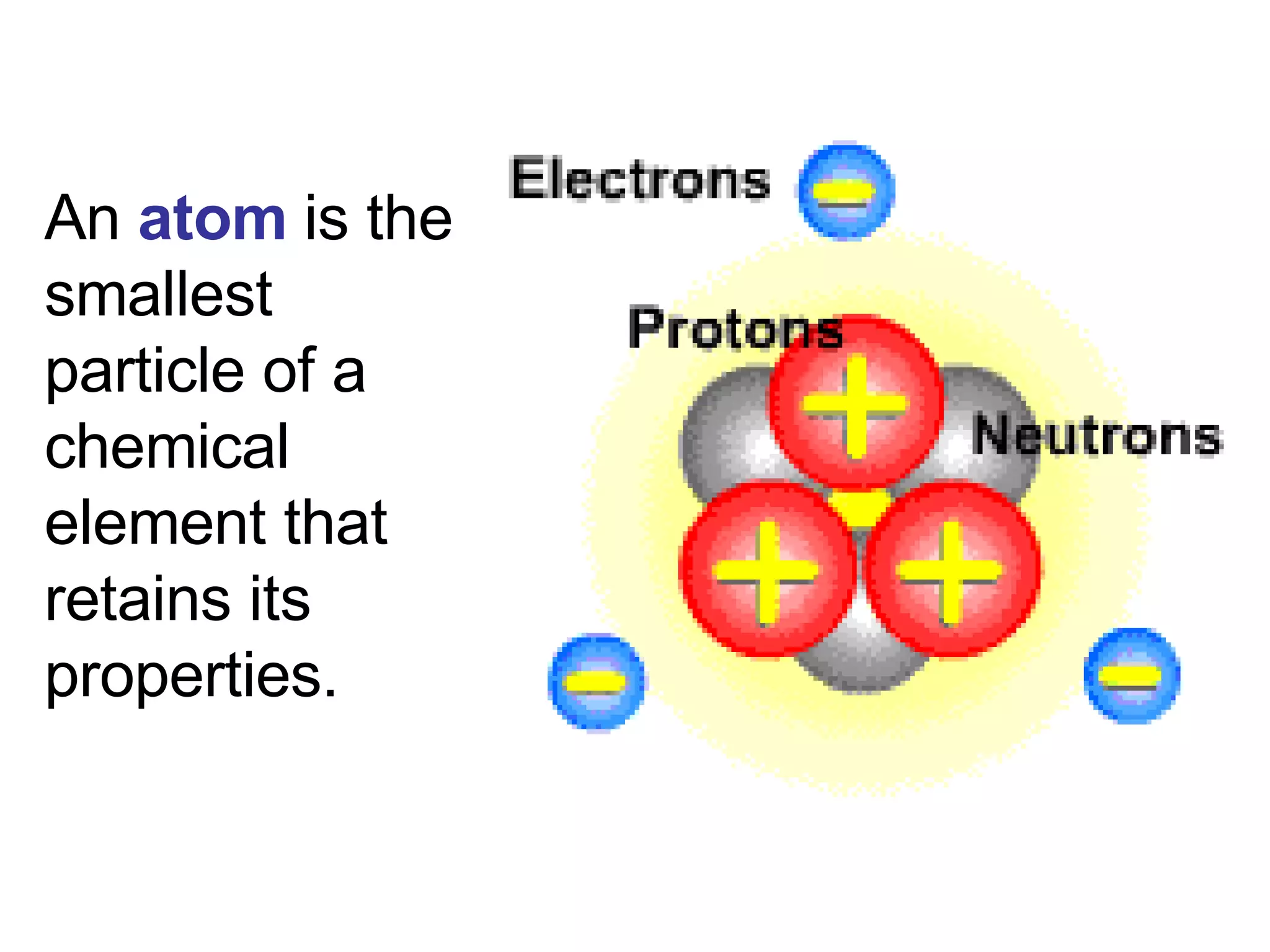
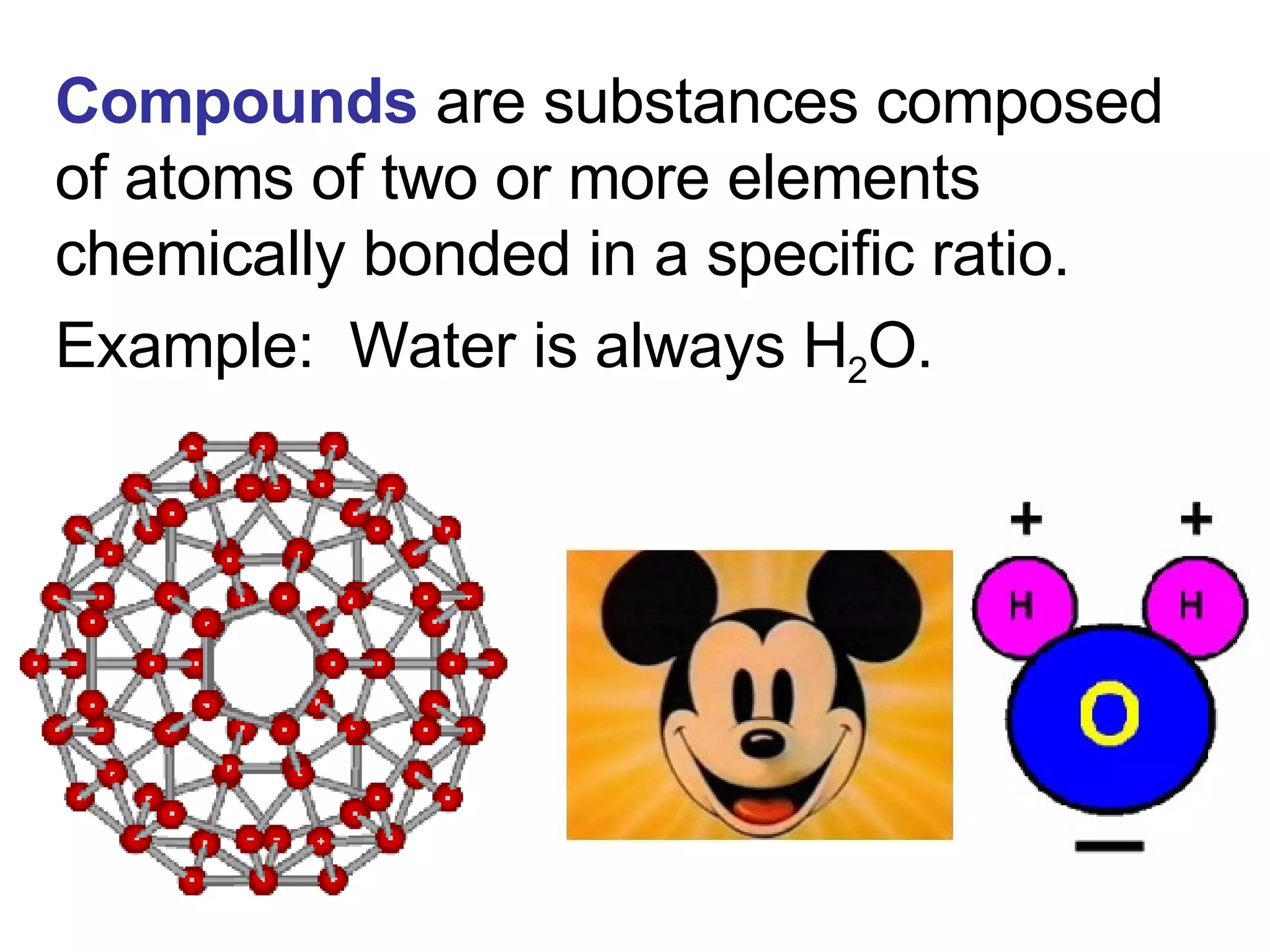
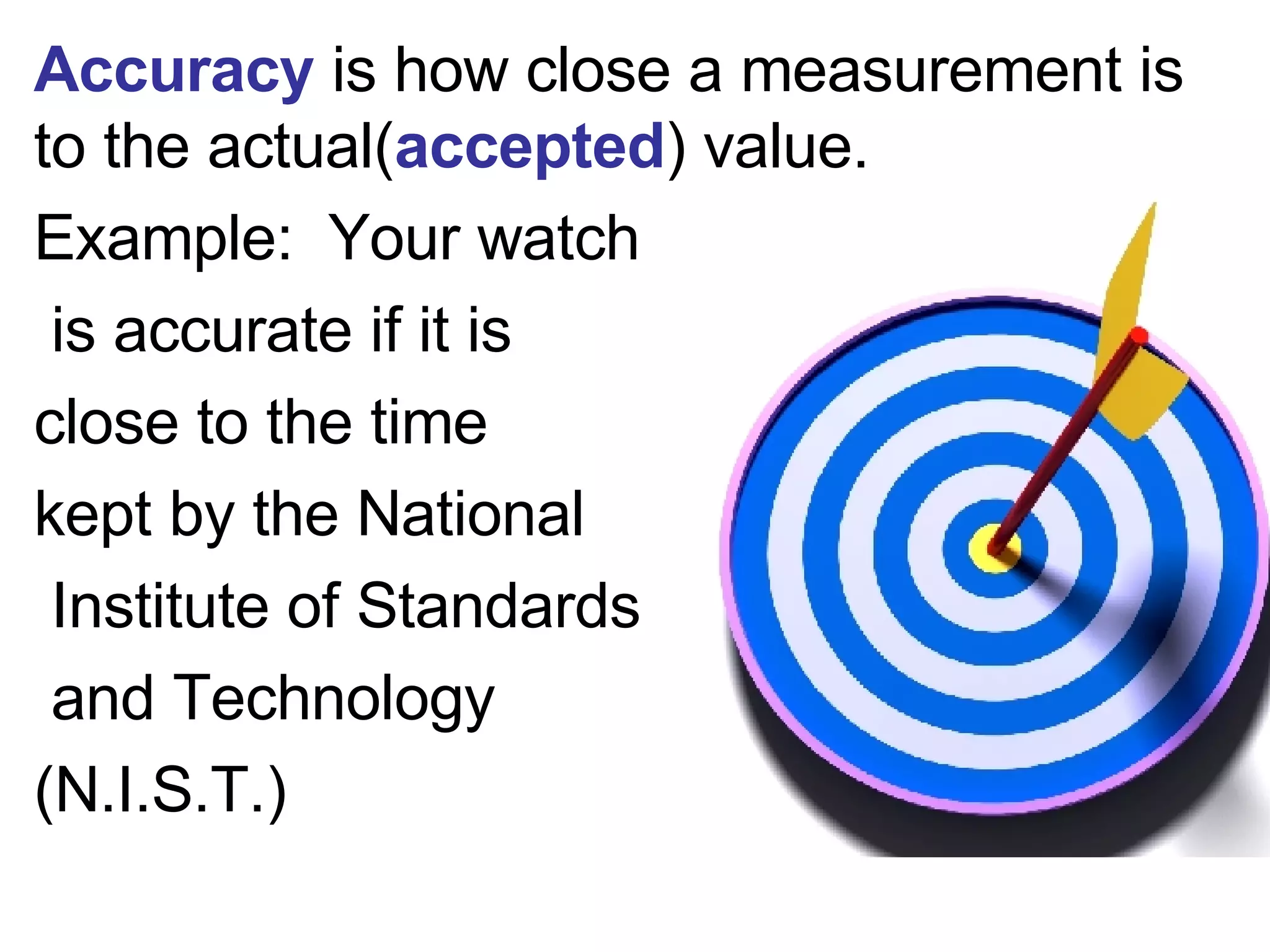
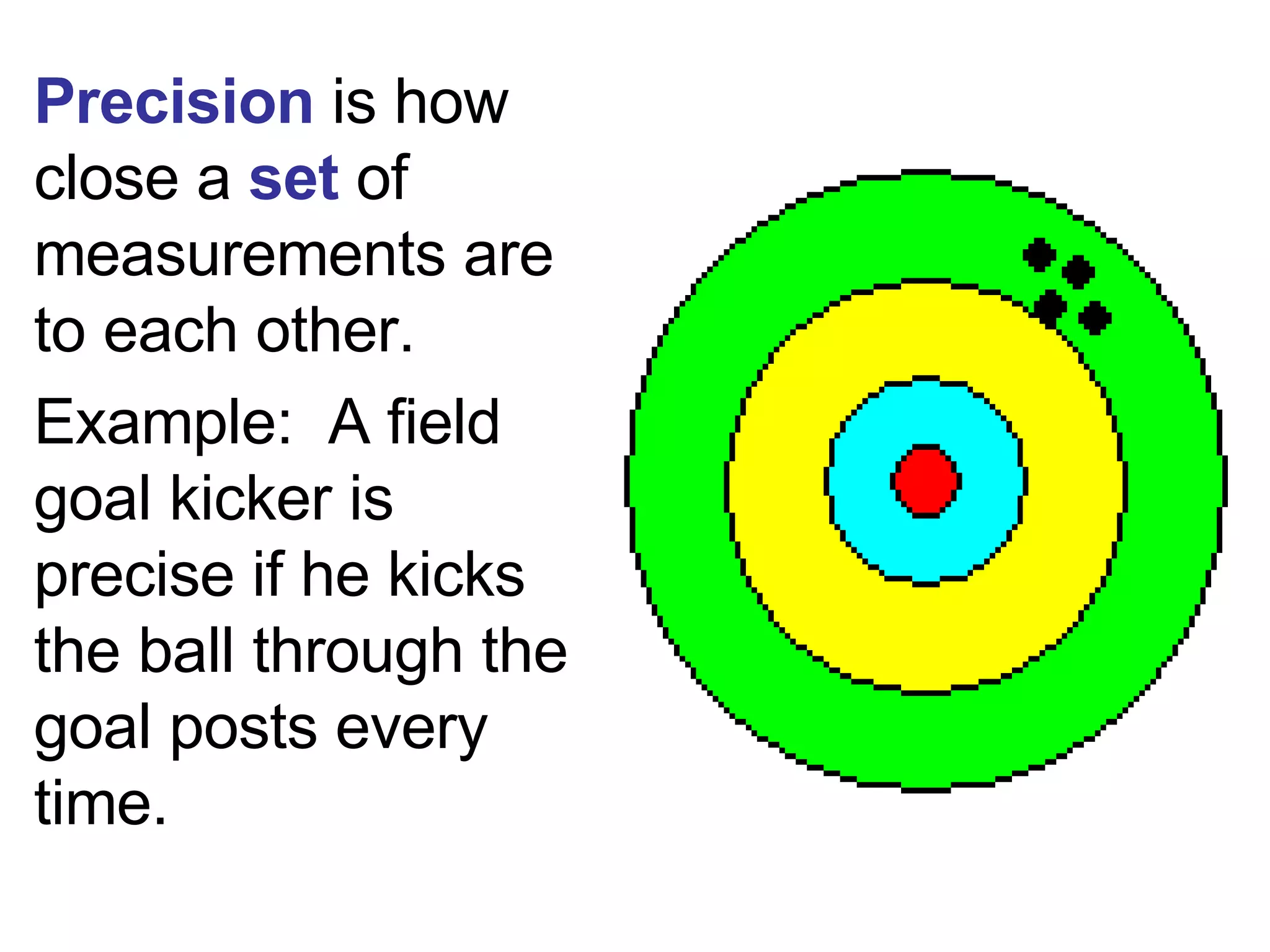
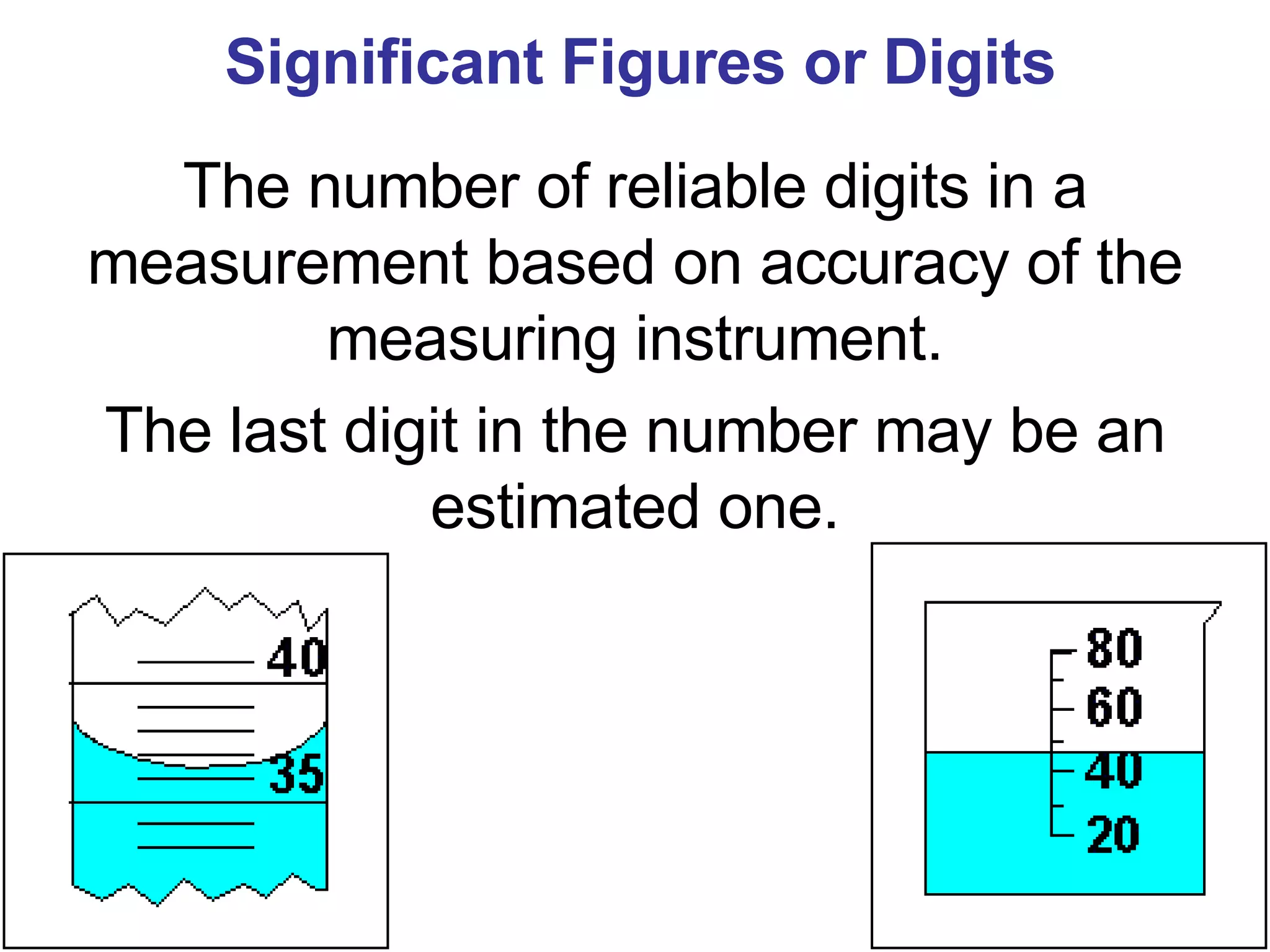
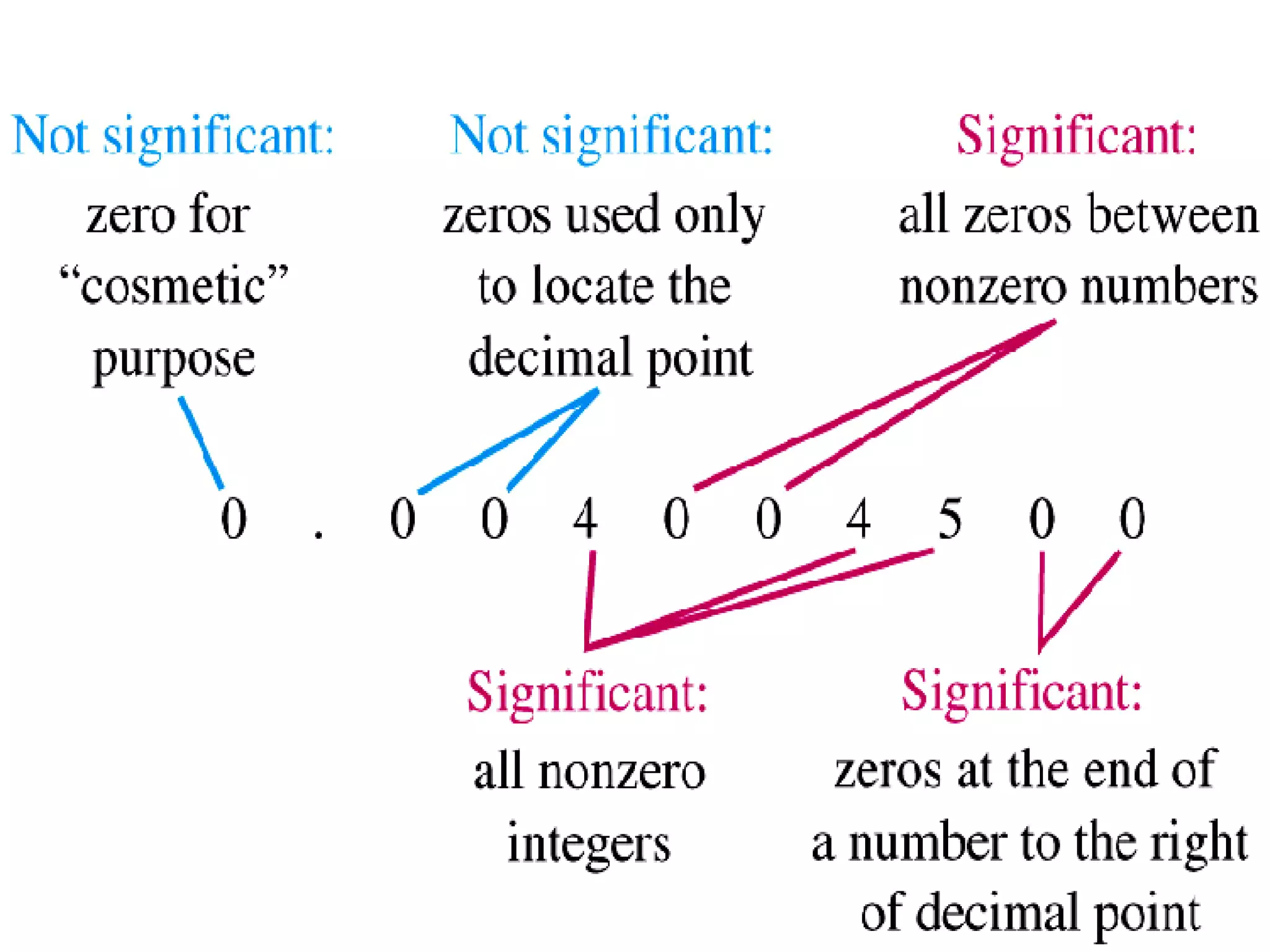
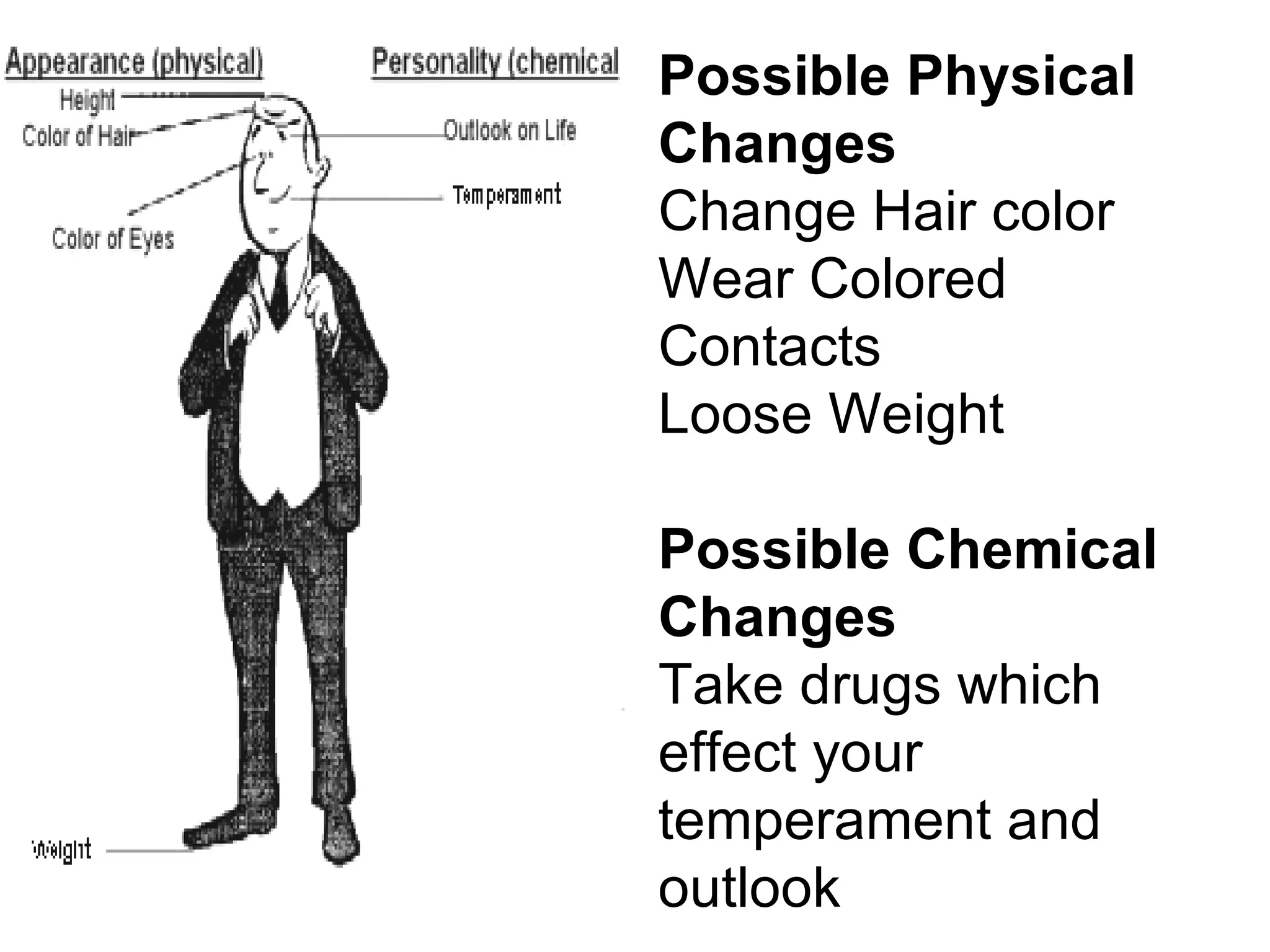
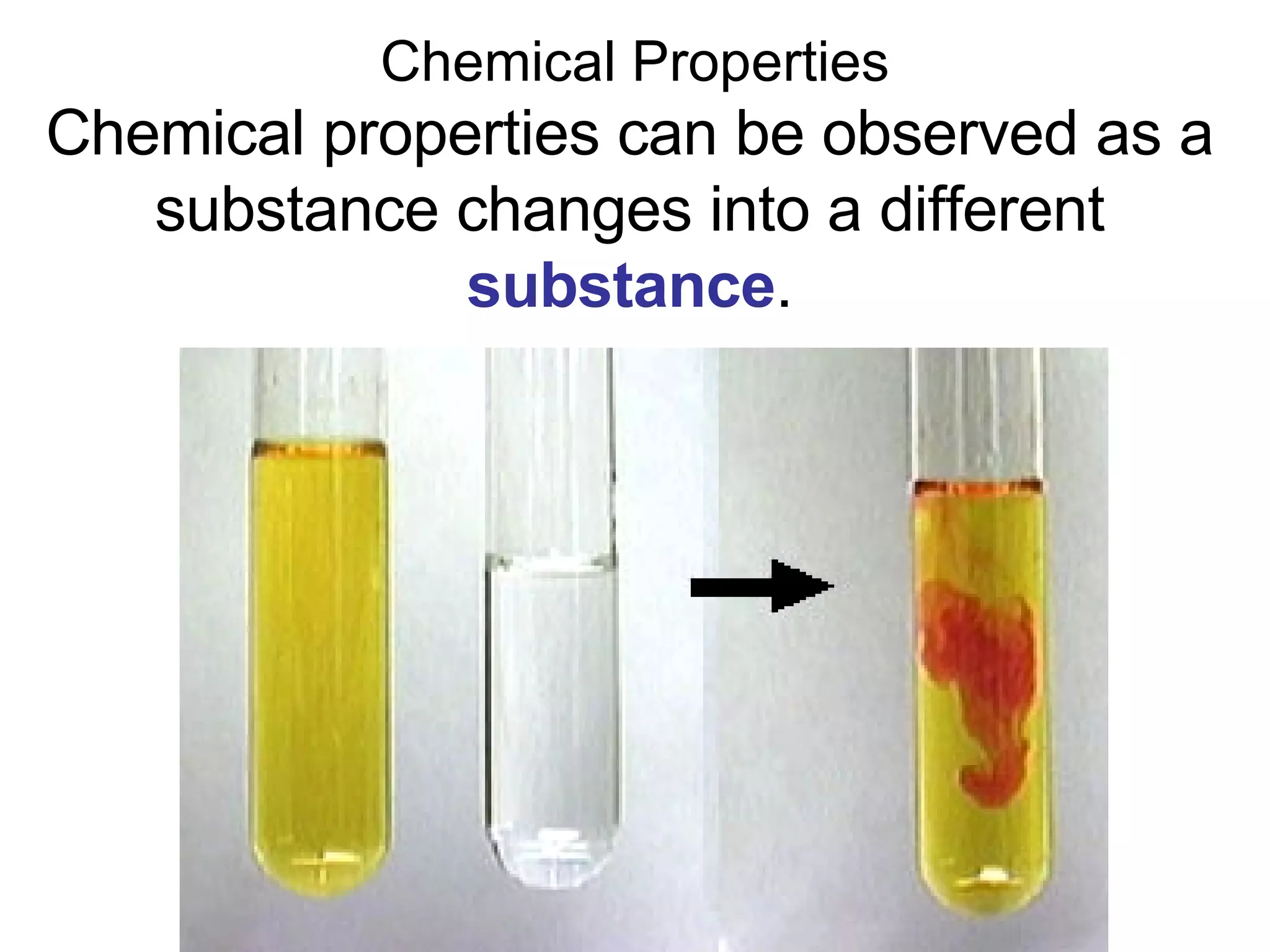
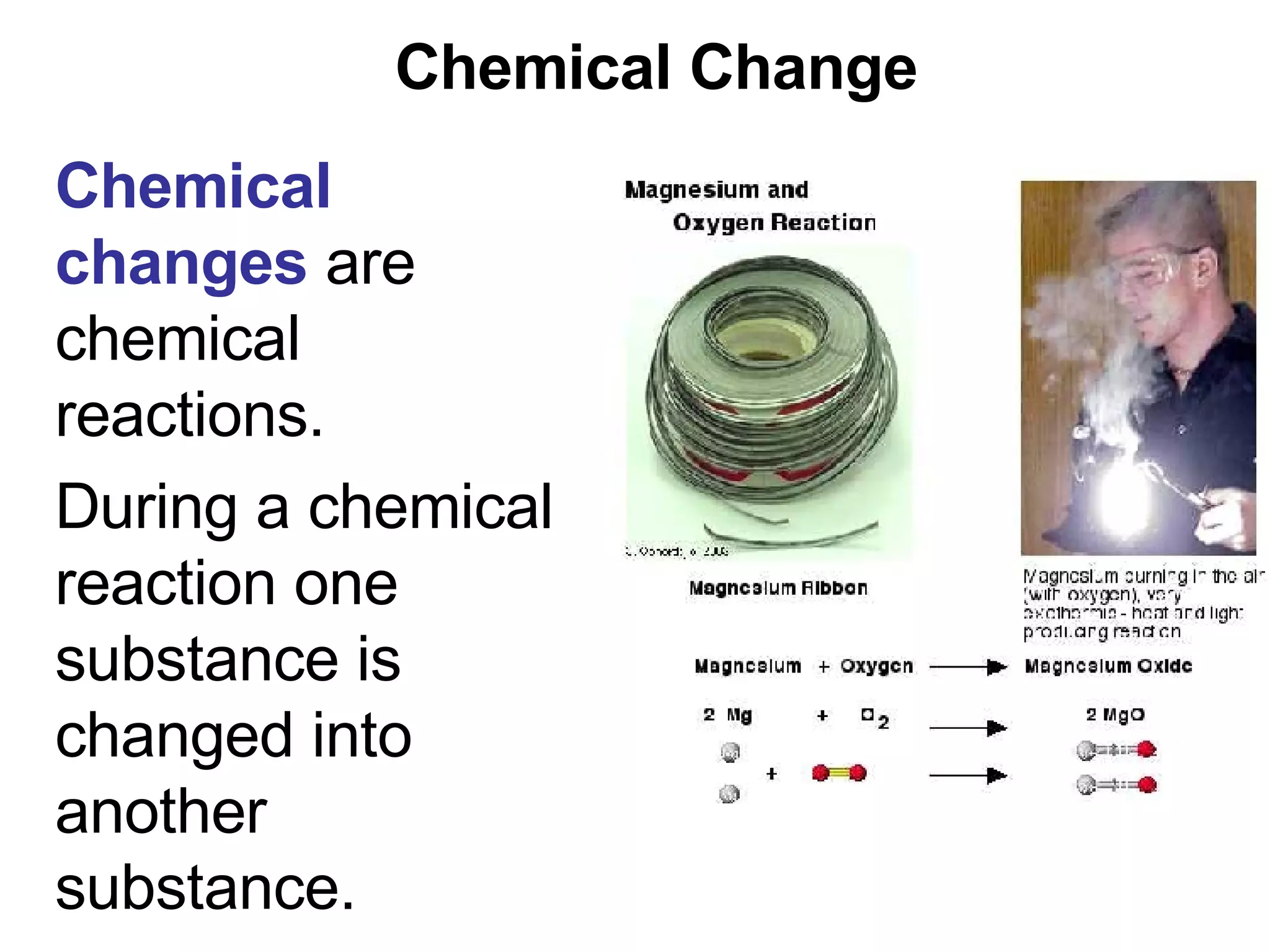
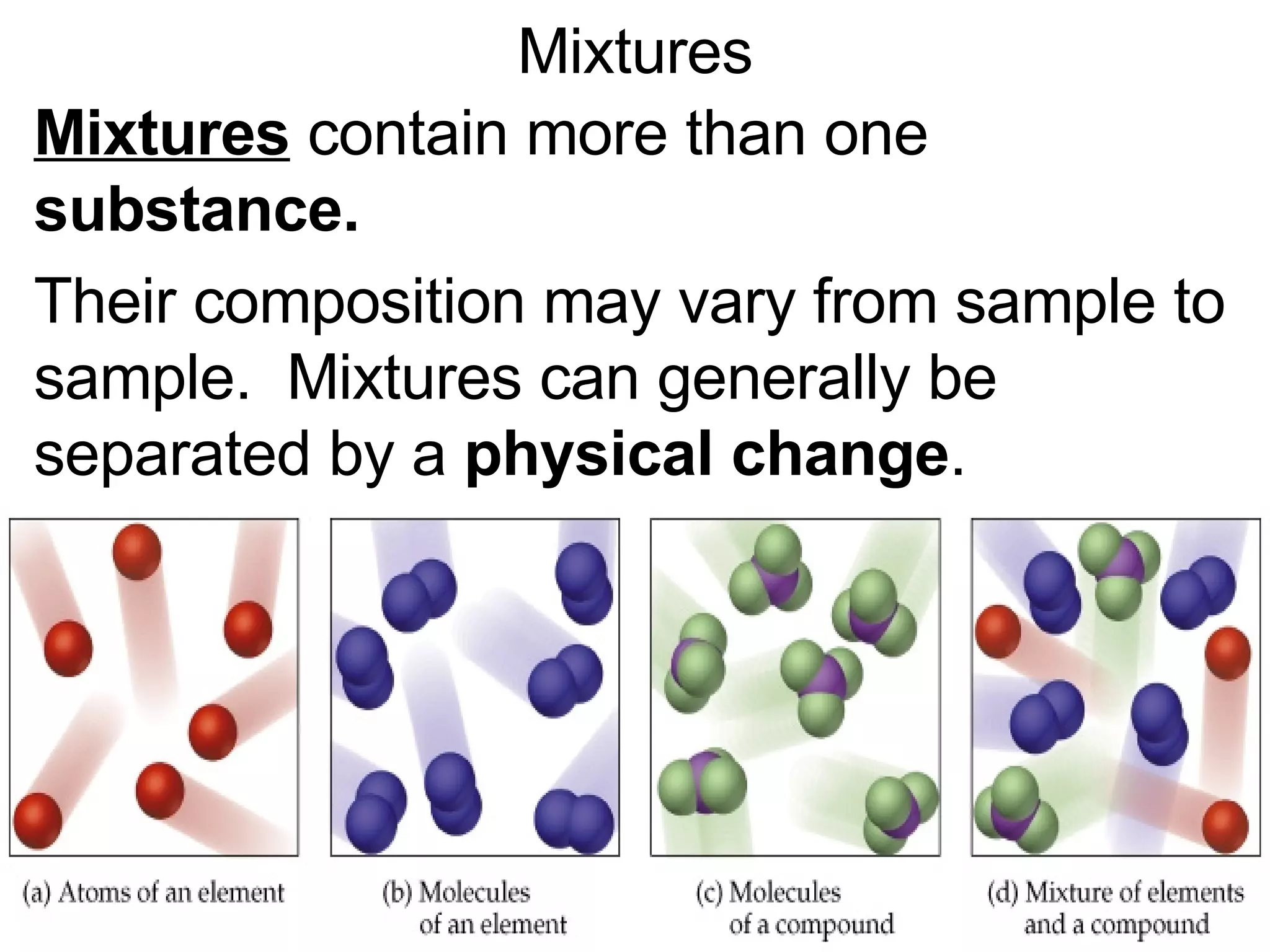
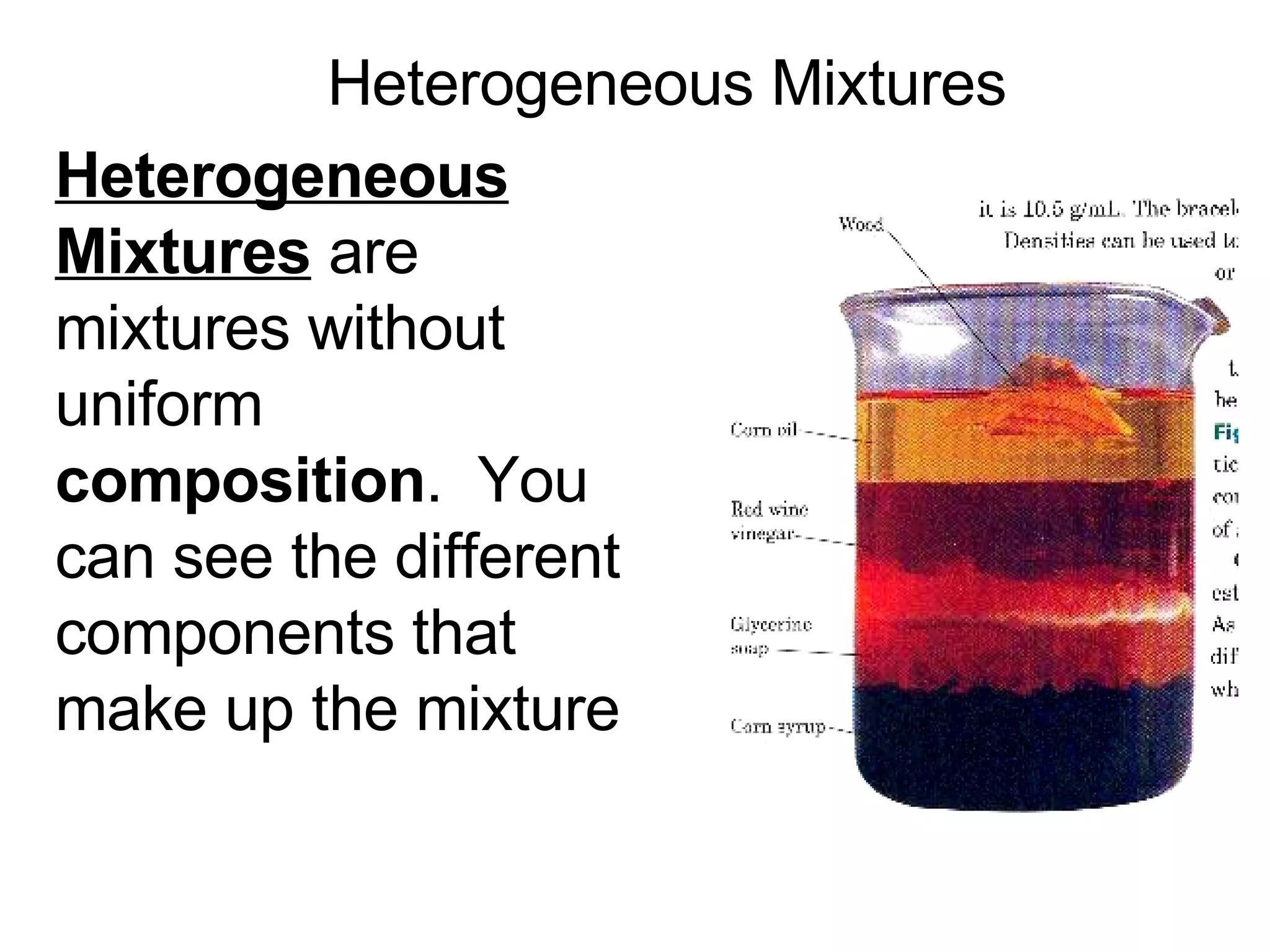
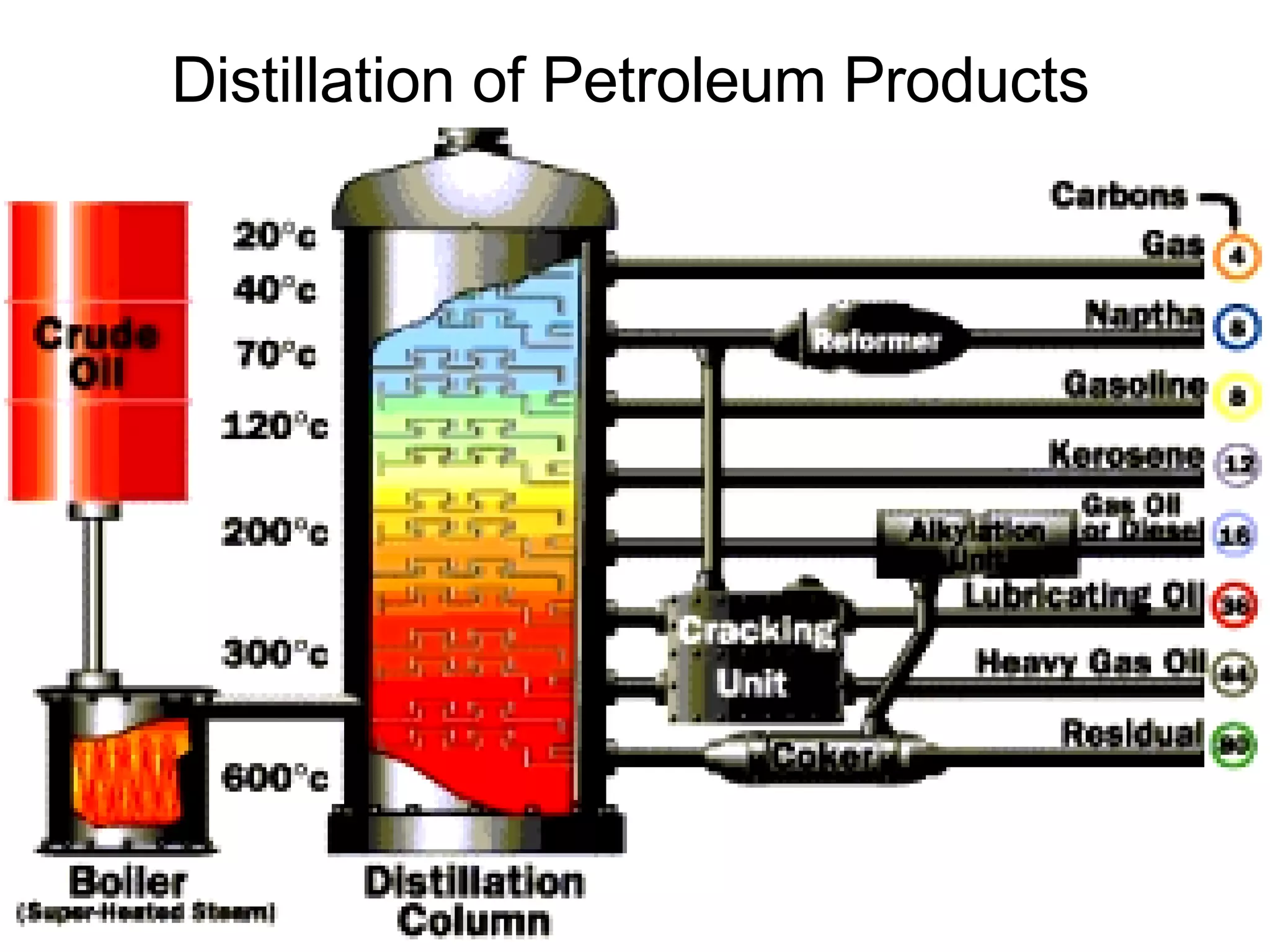
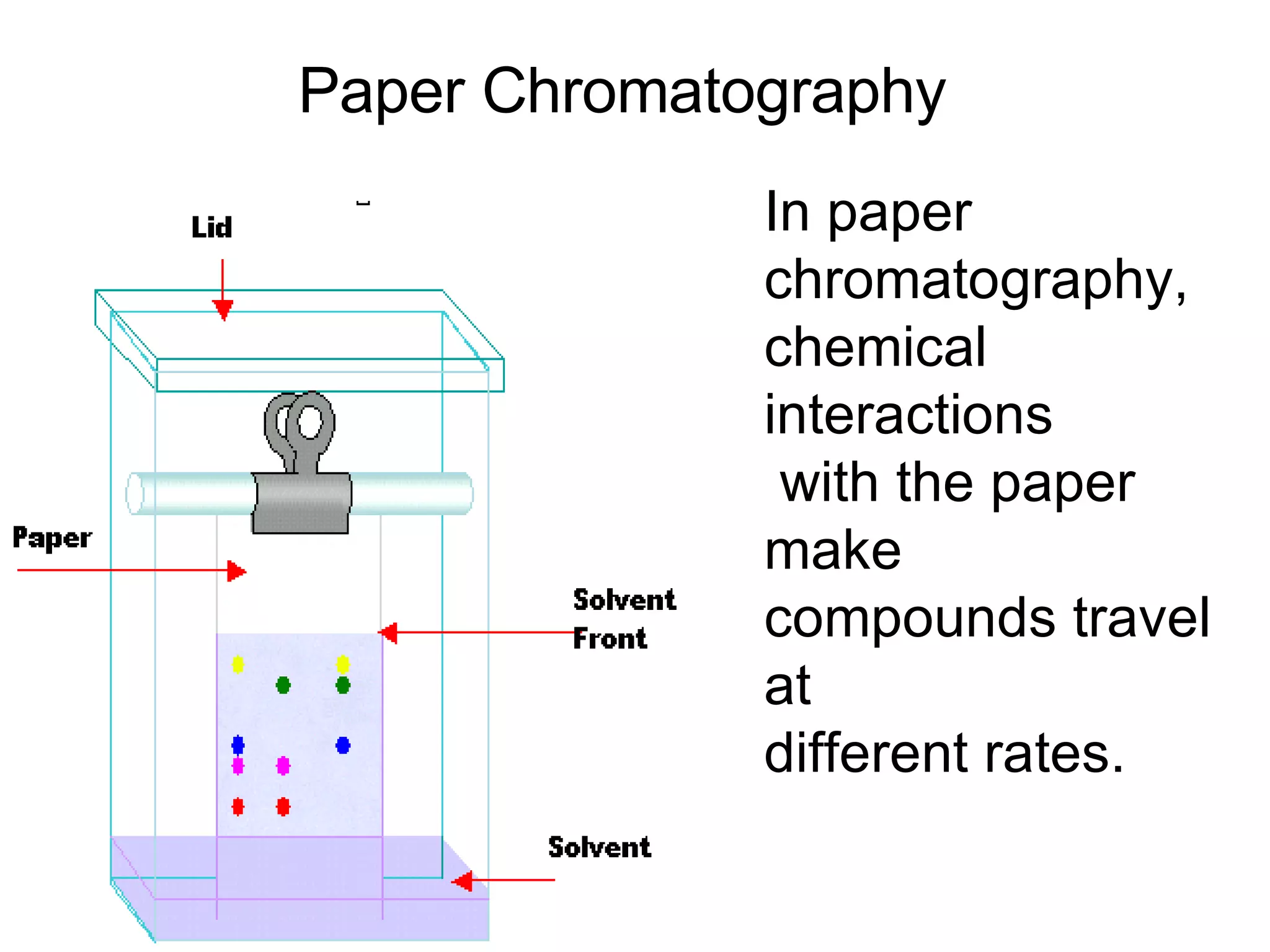
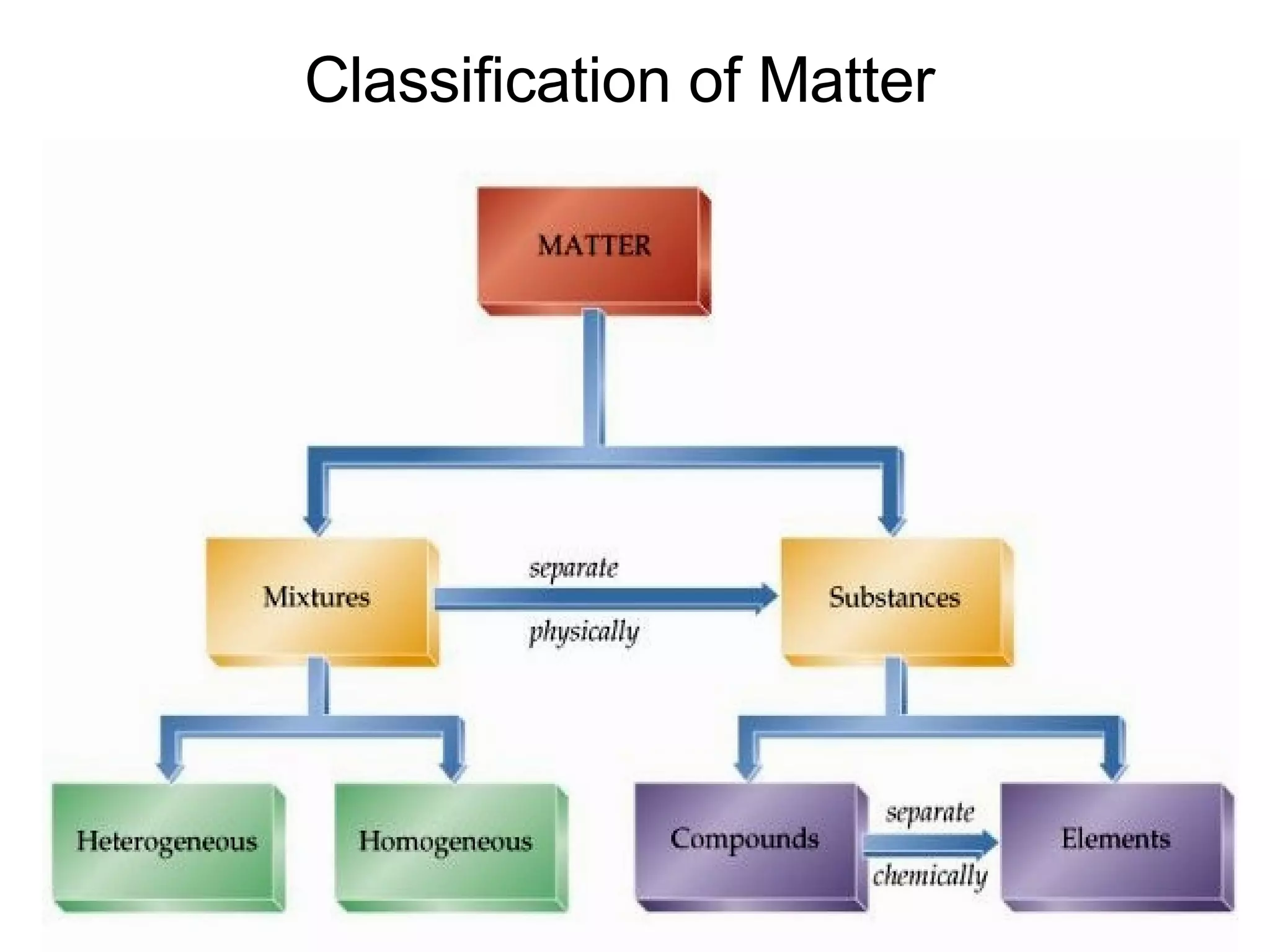
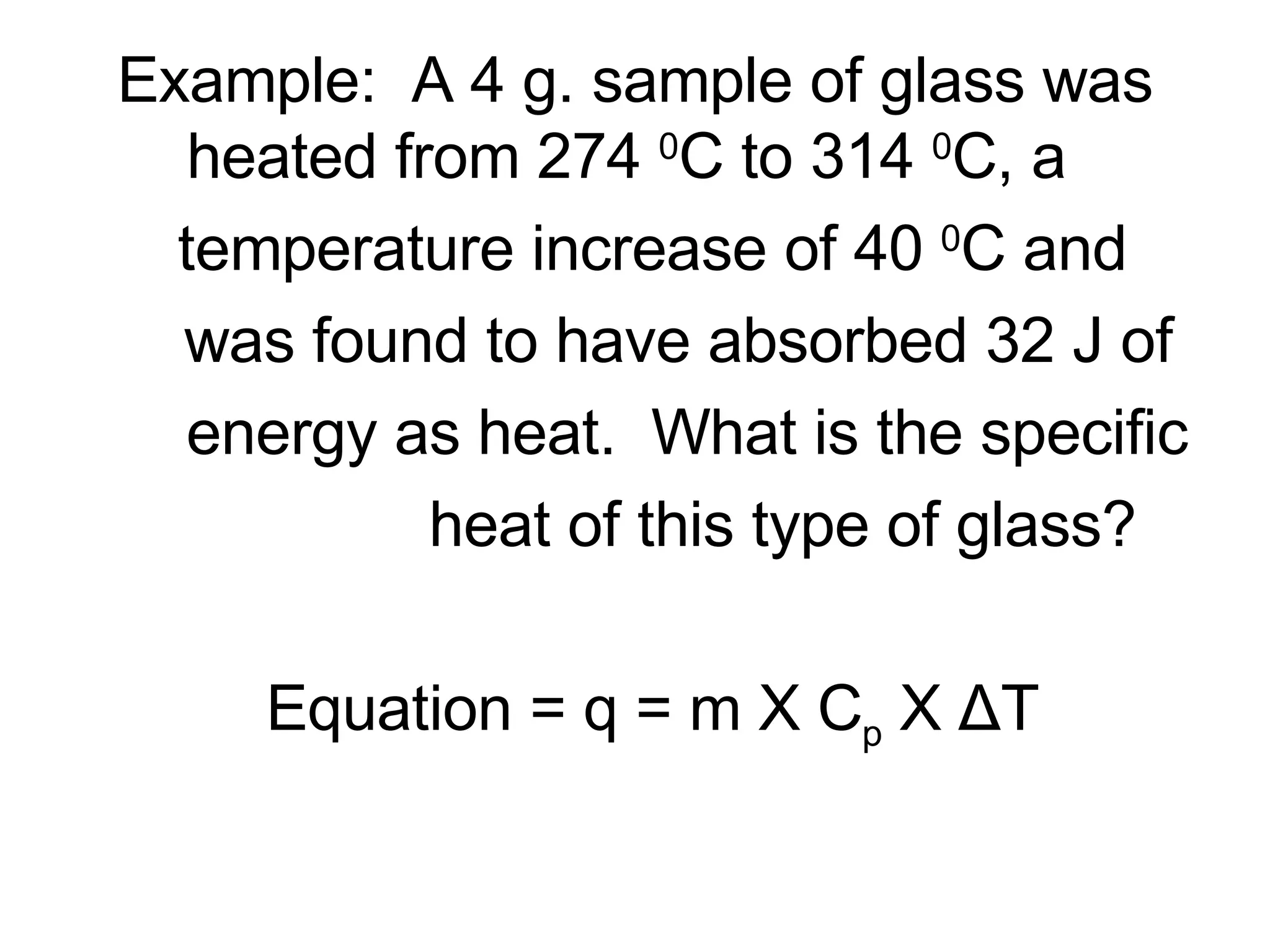This document provides an overview of key concepts in the unit on matter, including:
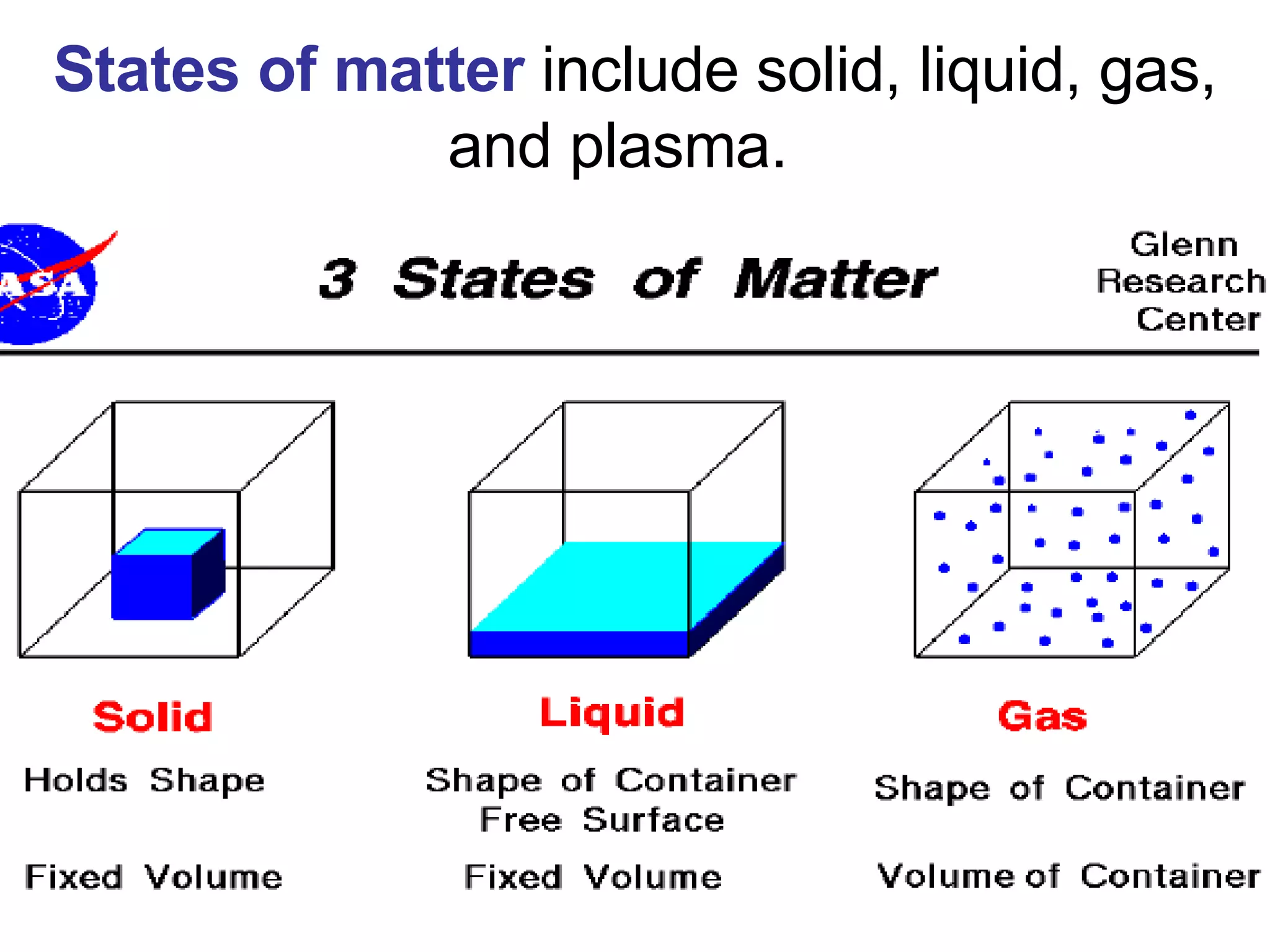
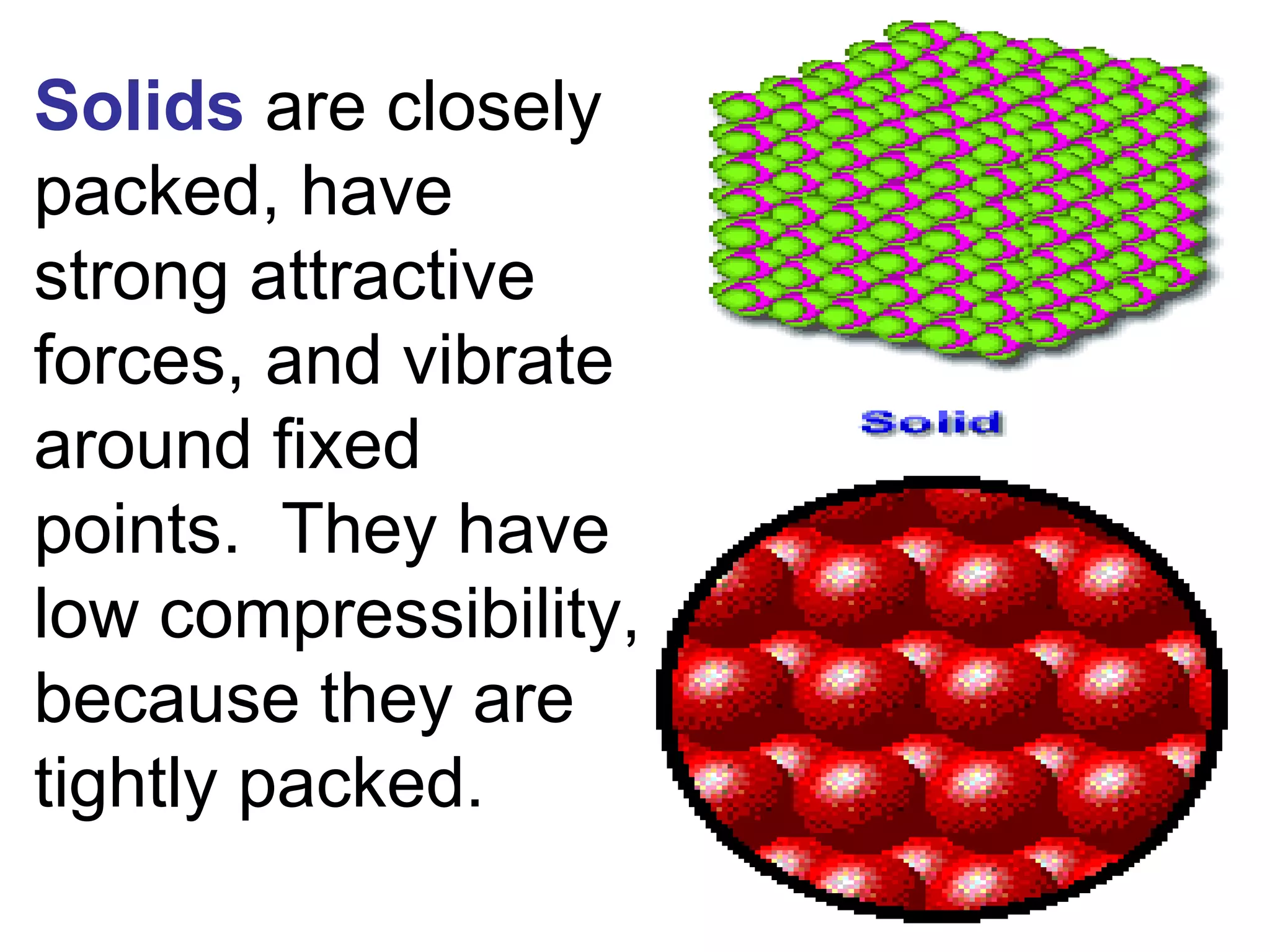
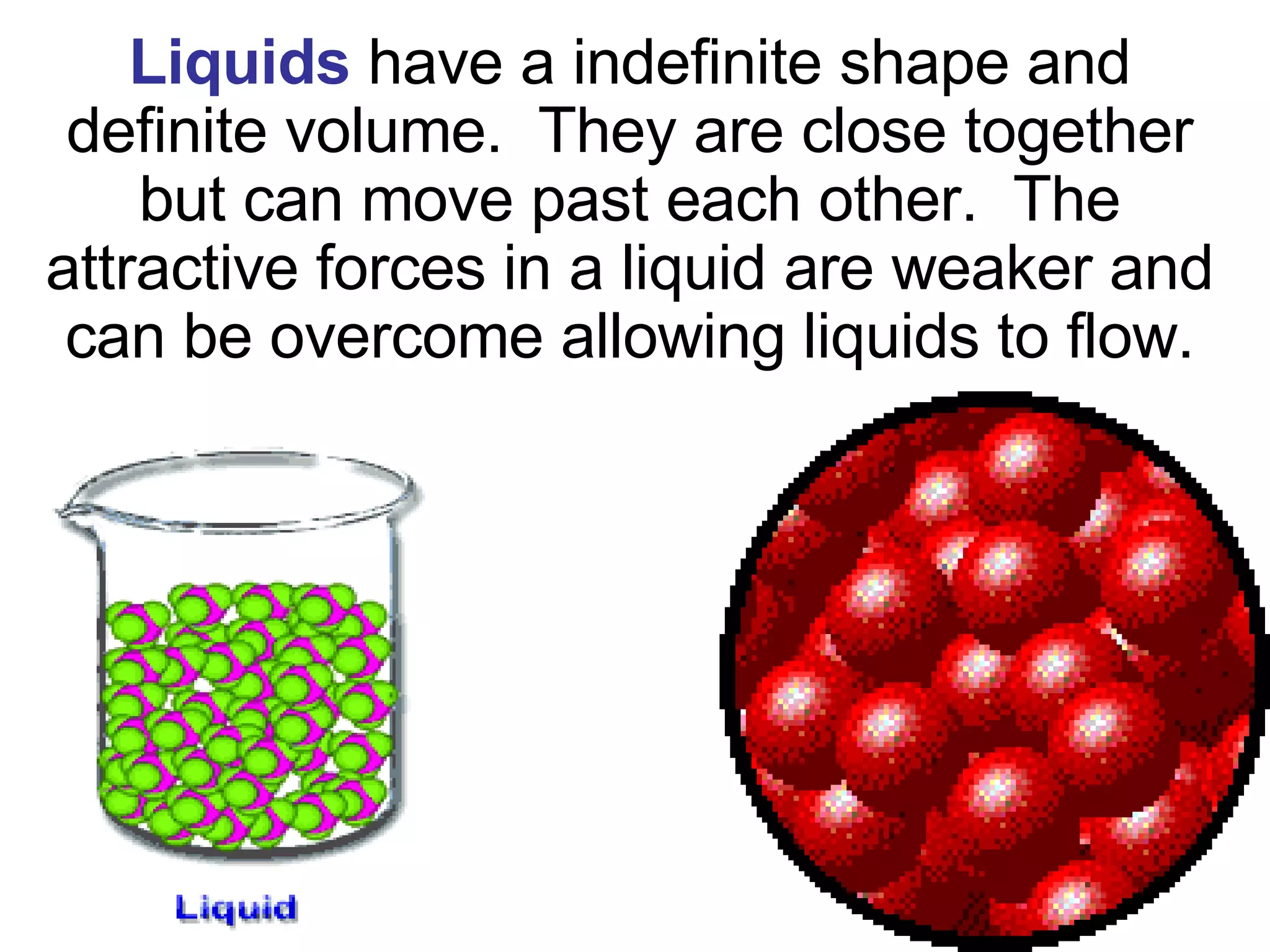
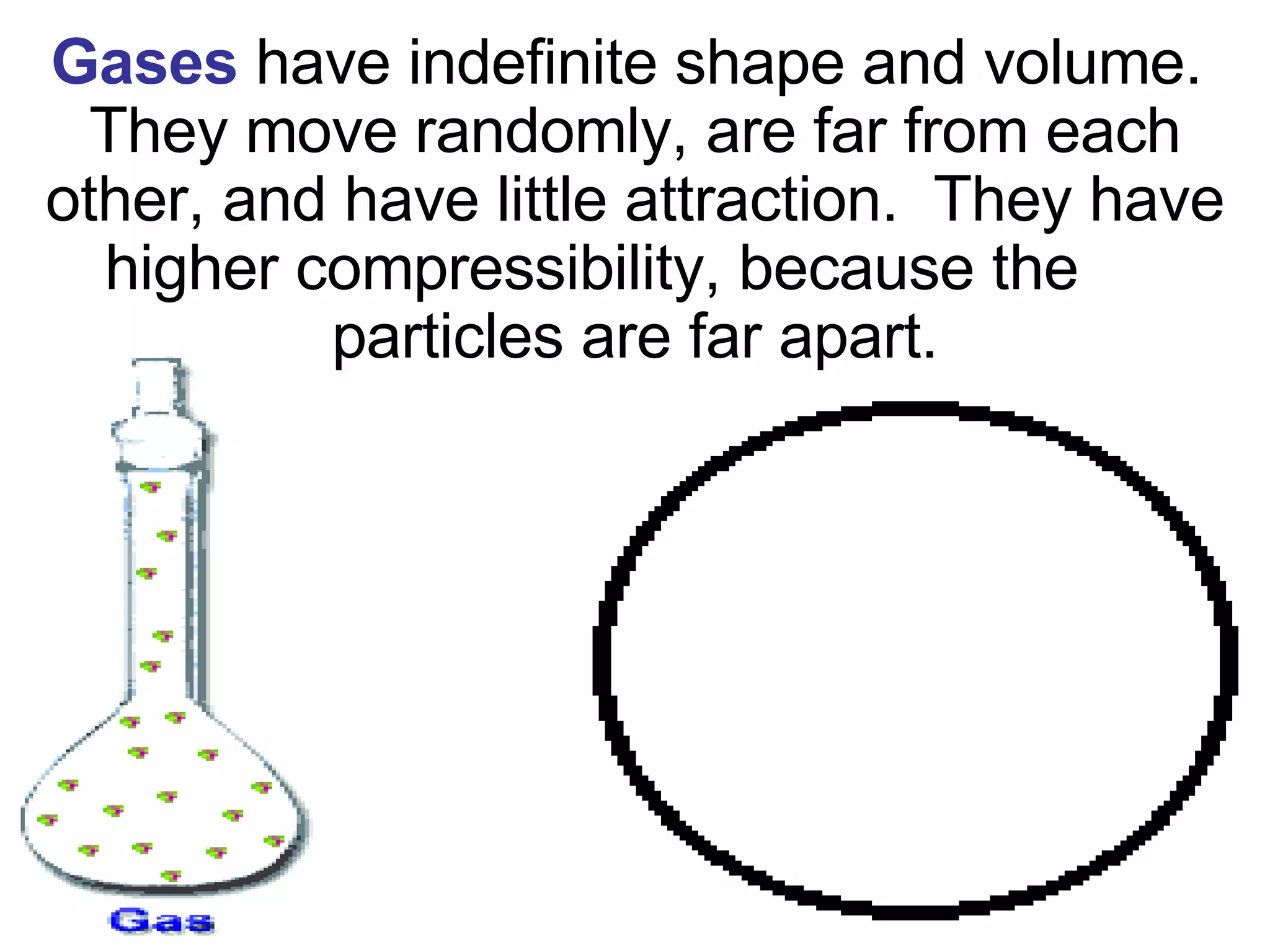
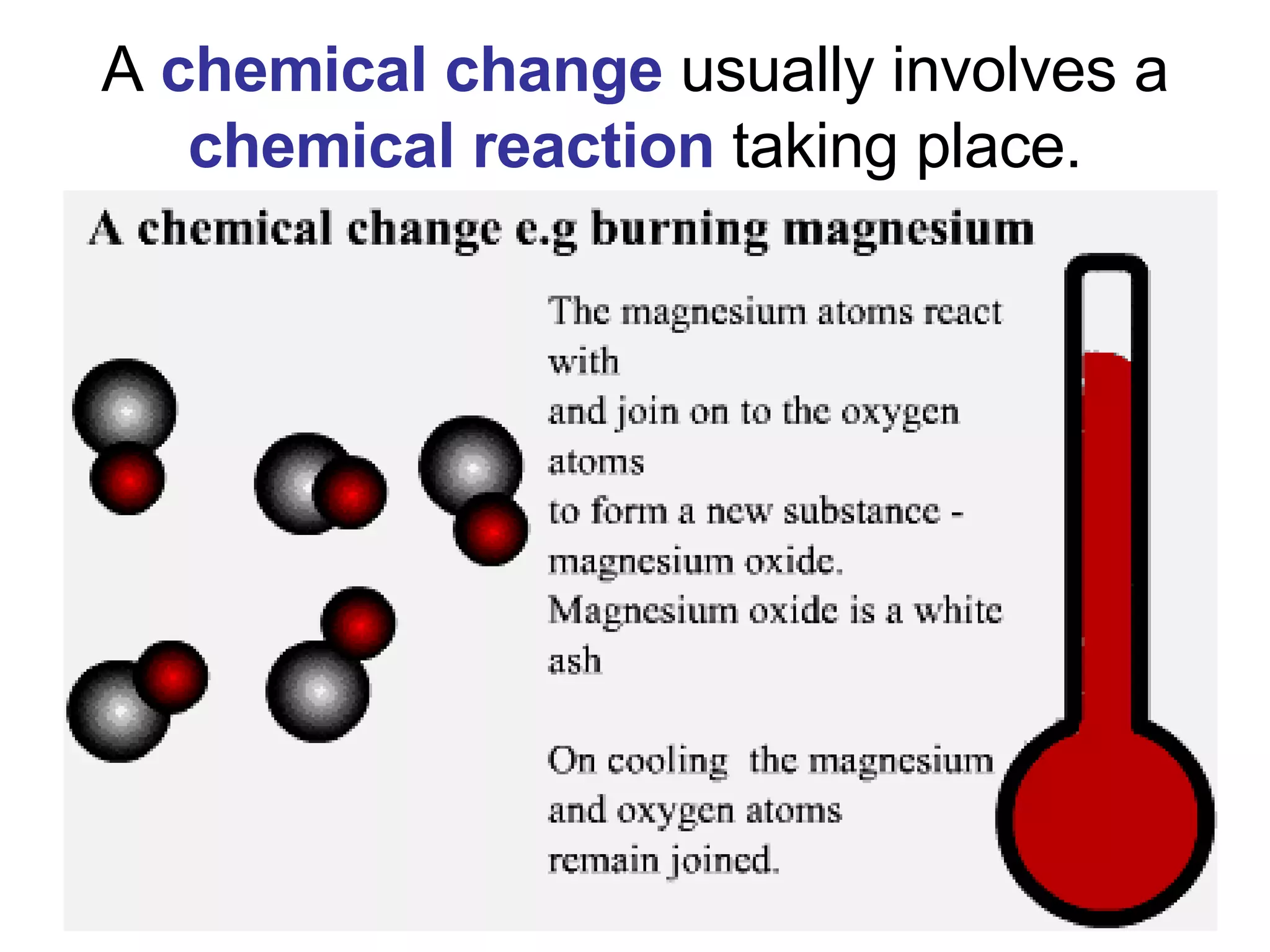
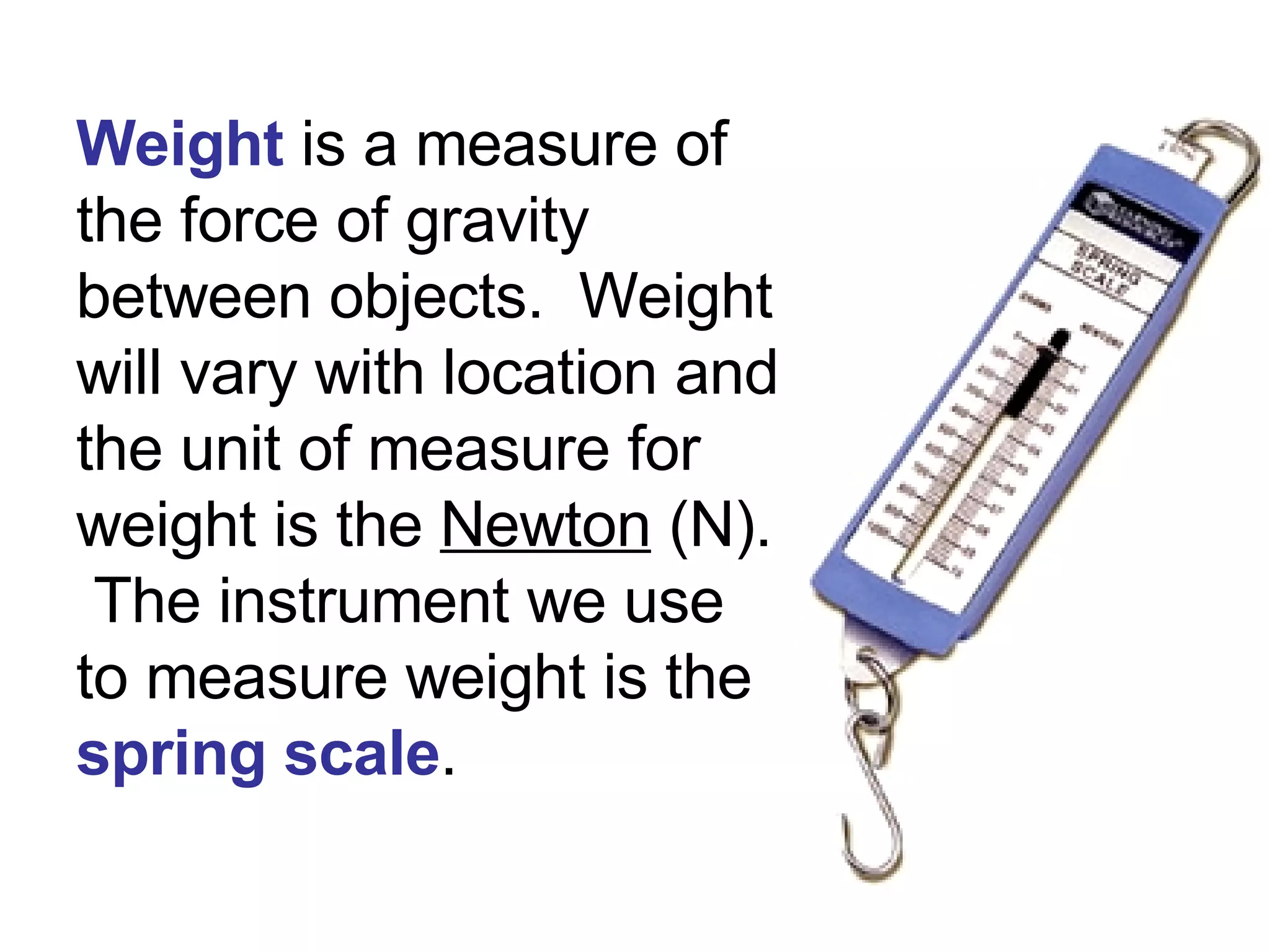
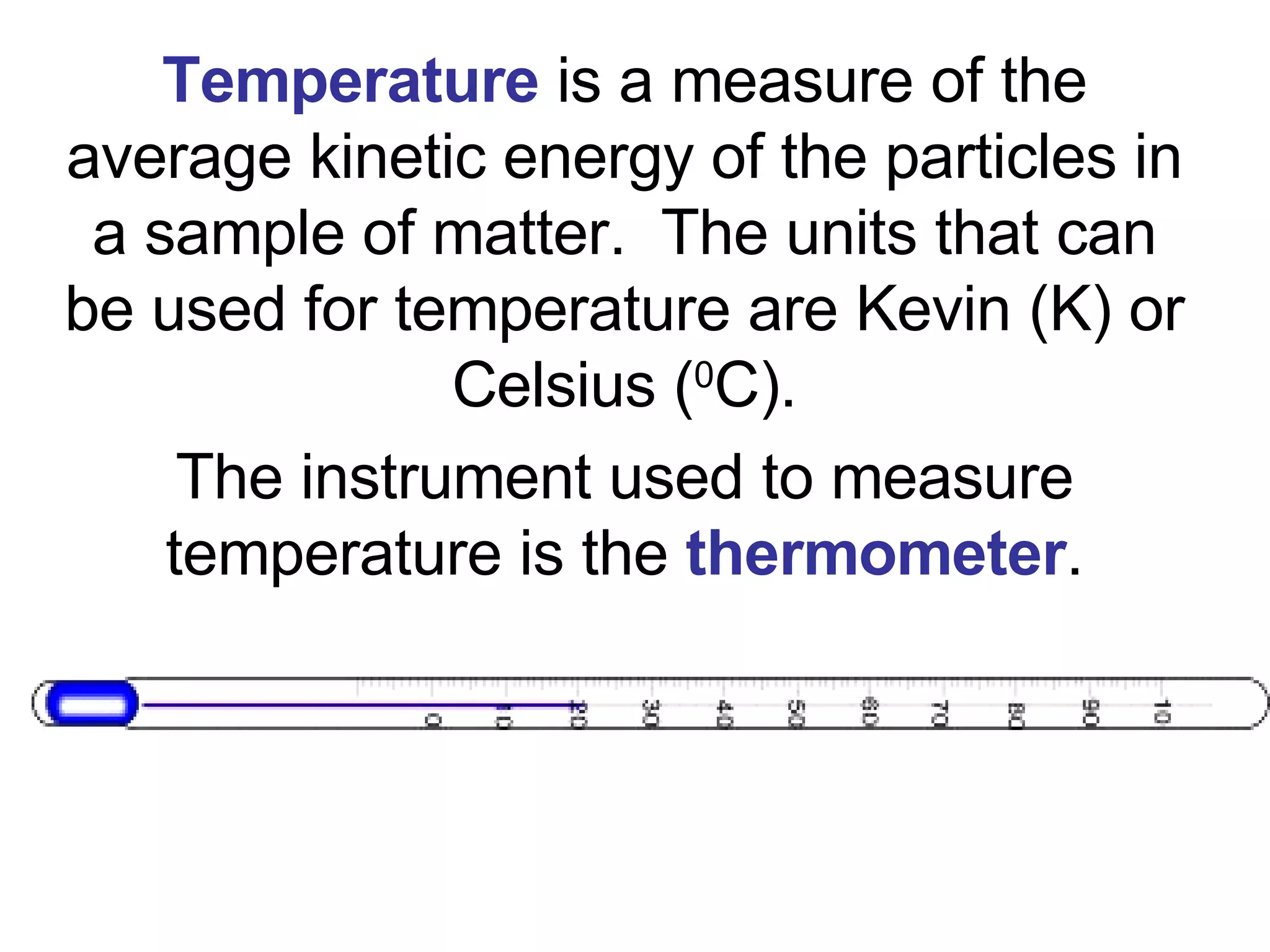
1. It defines matter as anything that has mass and volume, and discusses the states of matter (solid, liquid, gas, plasma), physical and chemical properties, and physical and chemical changes.
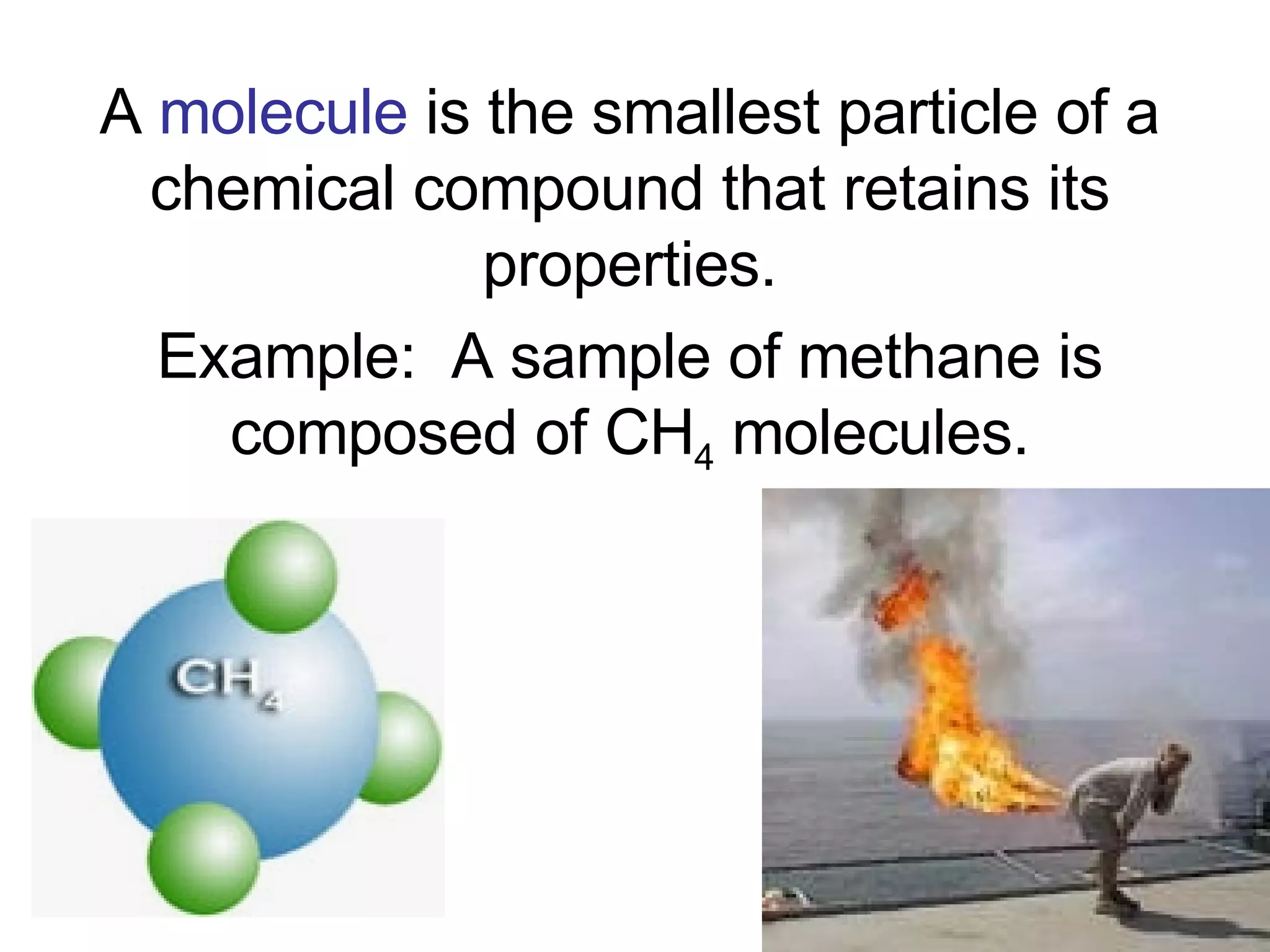
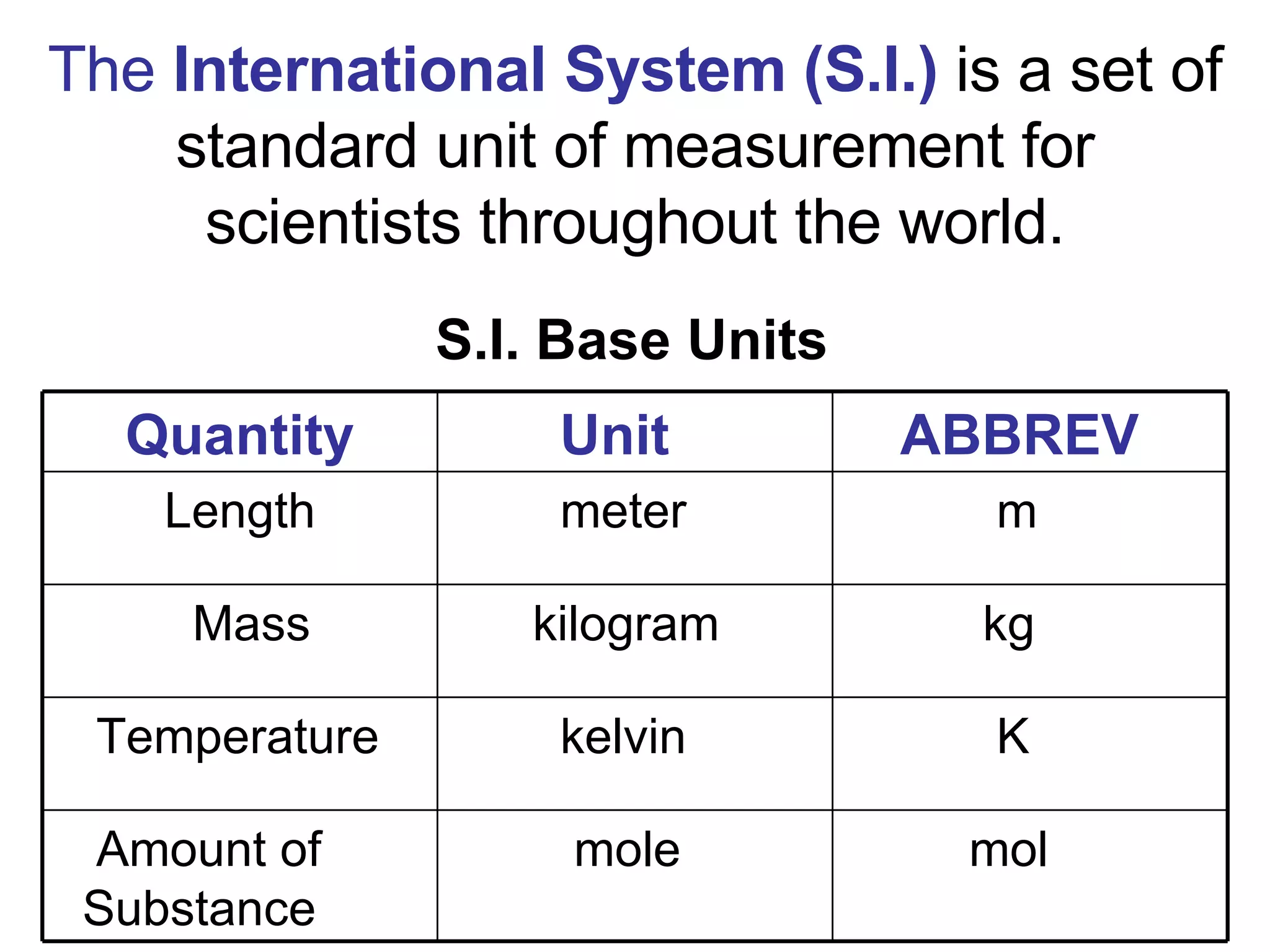
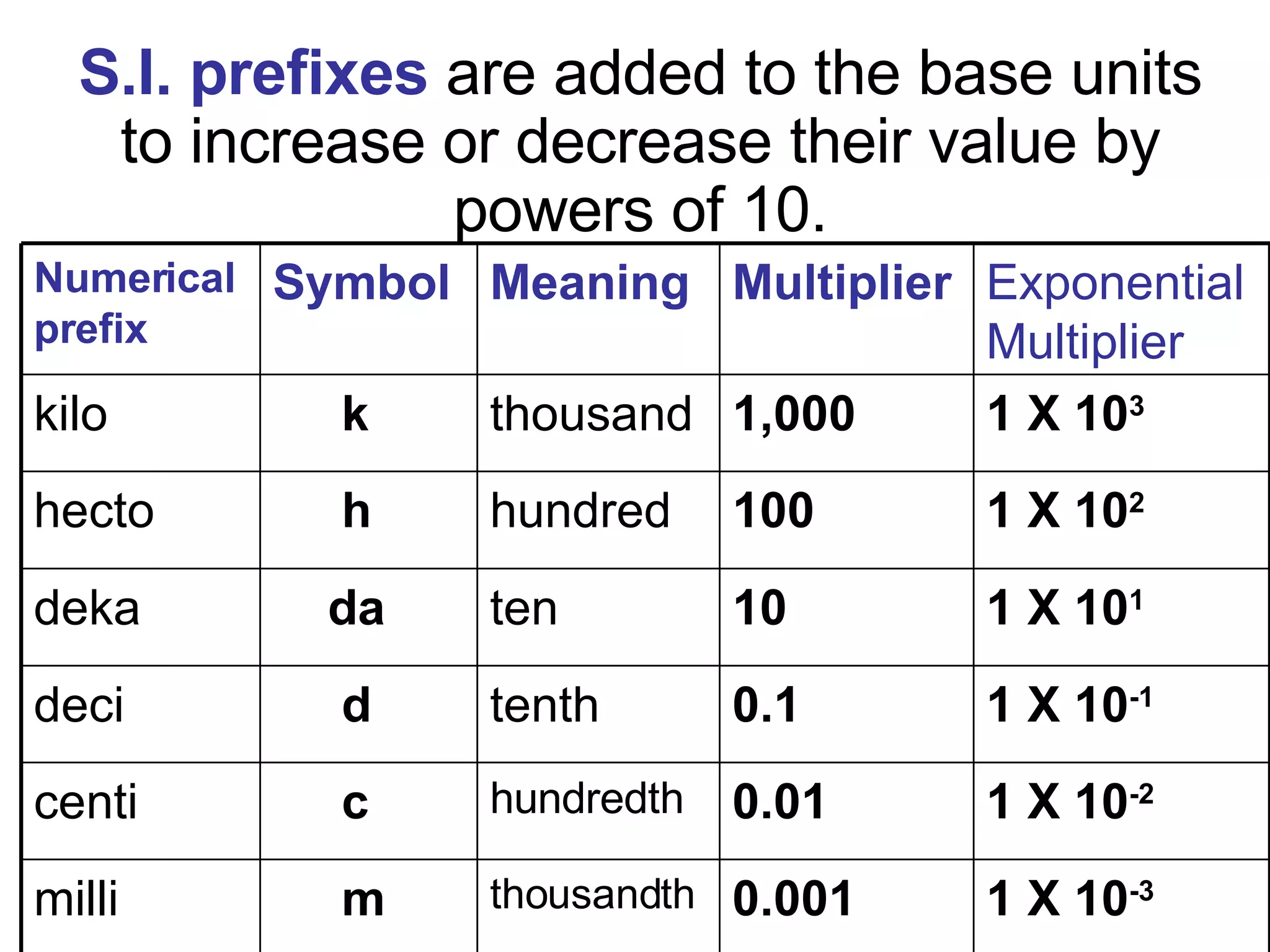
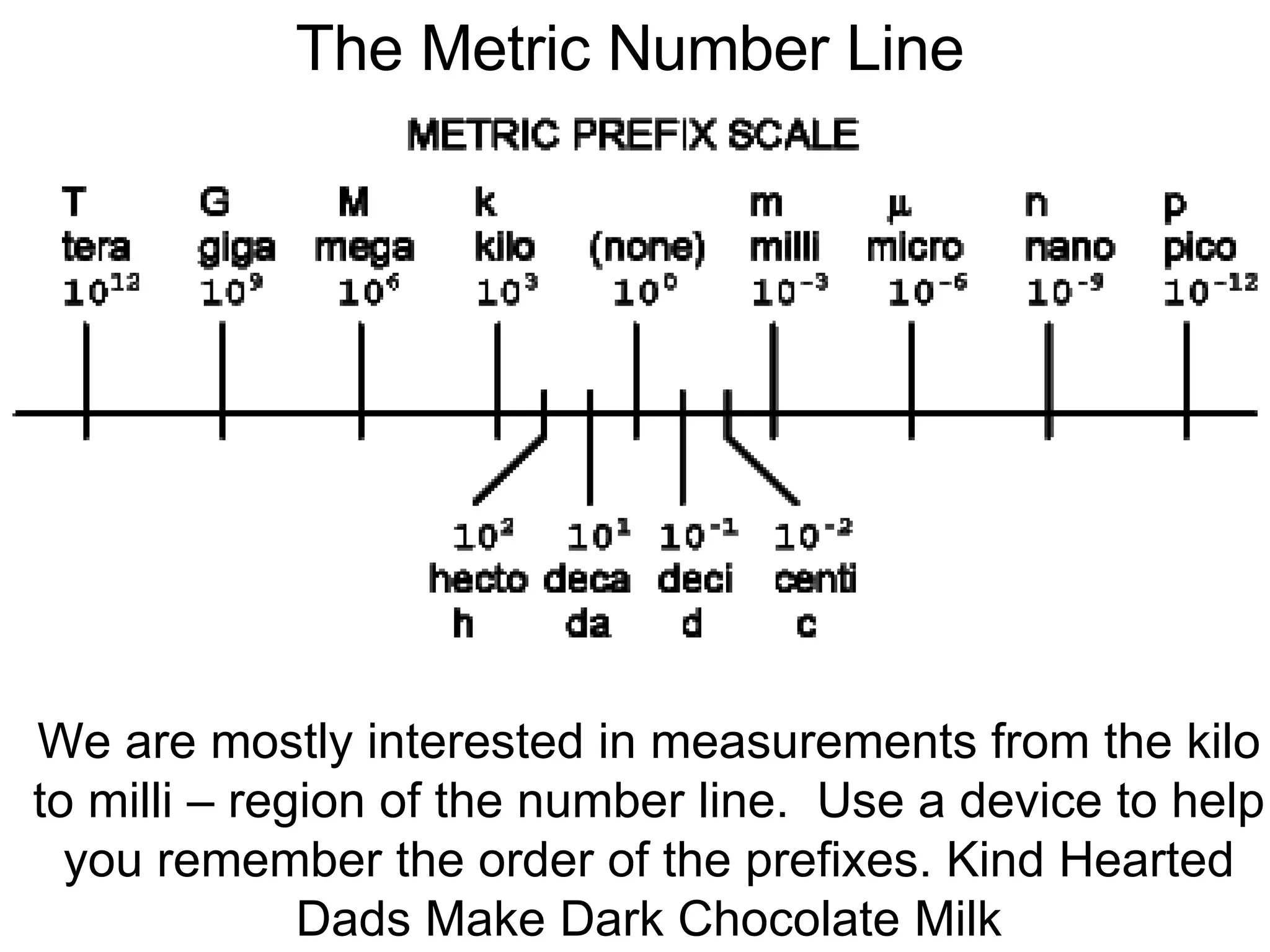
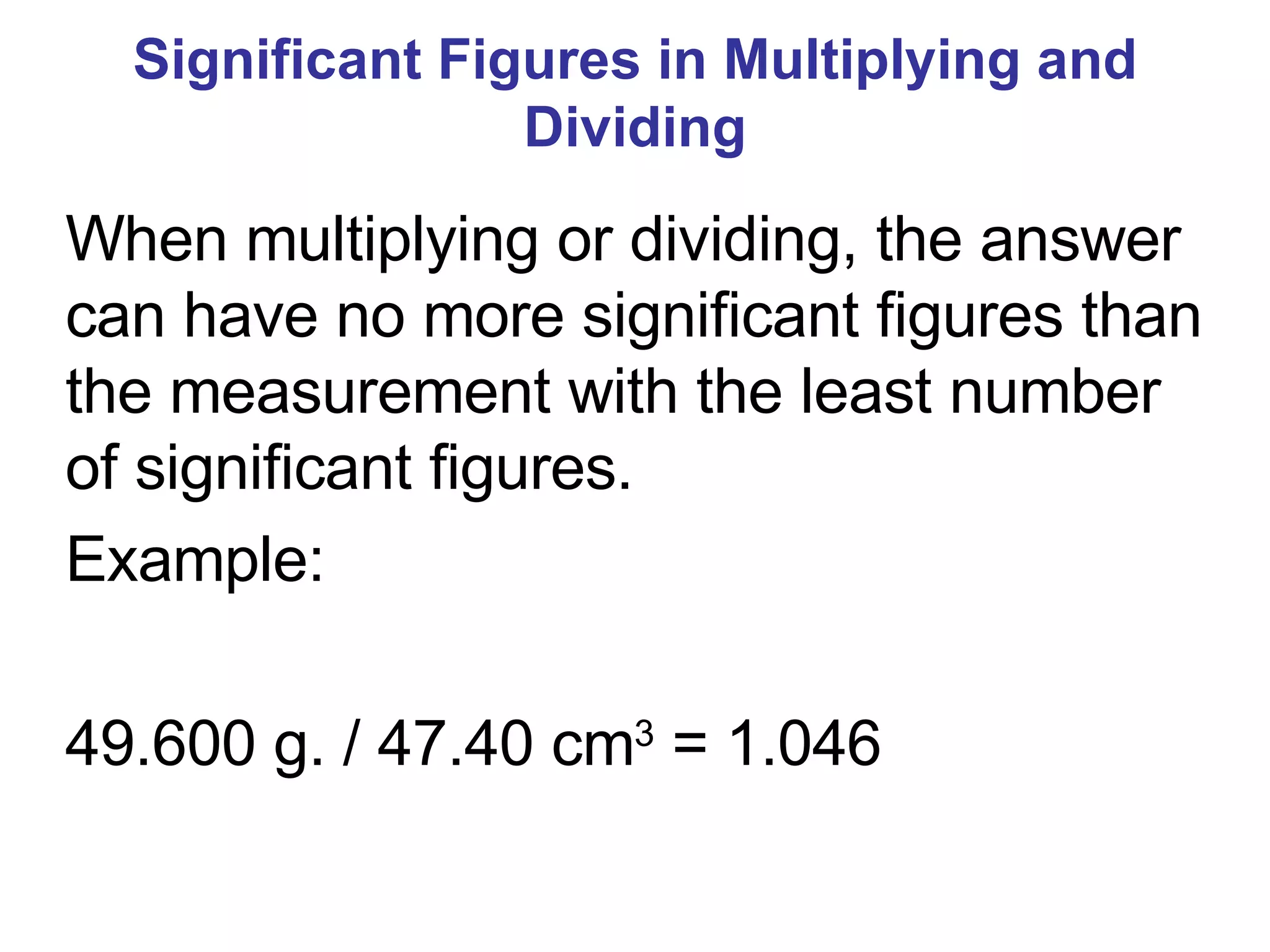
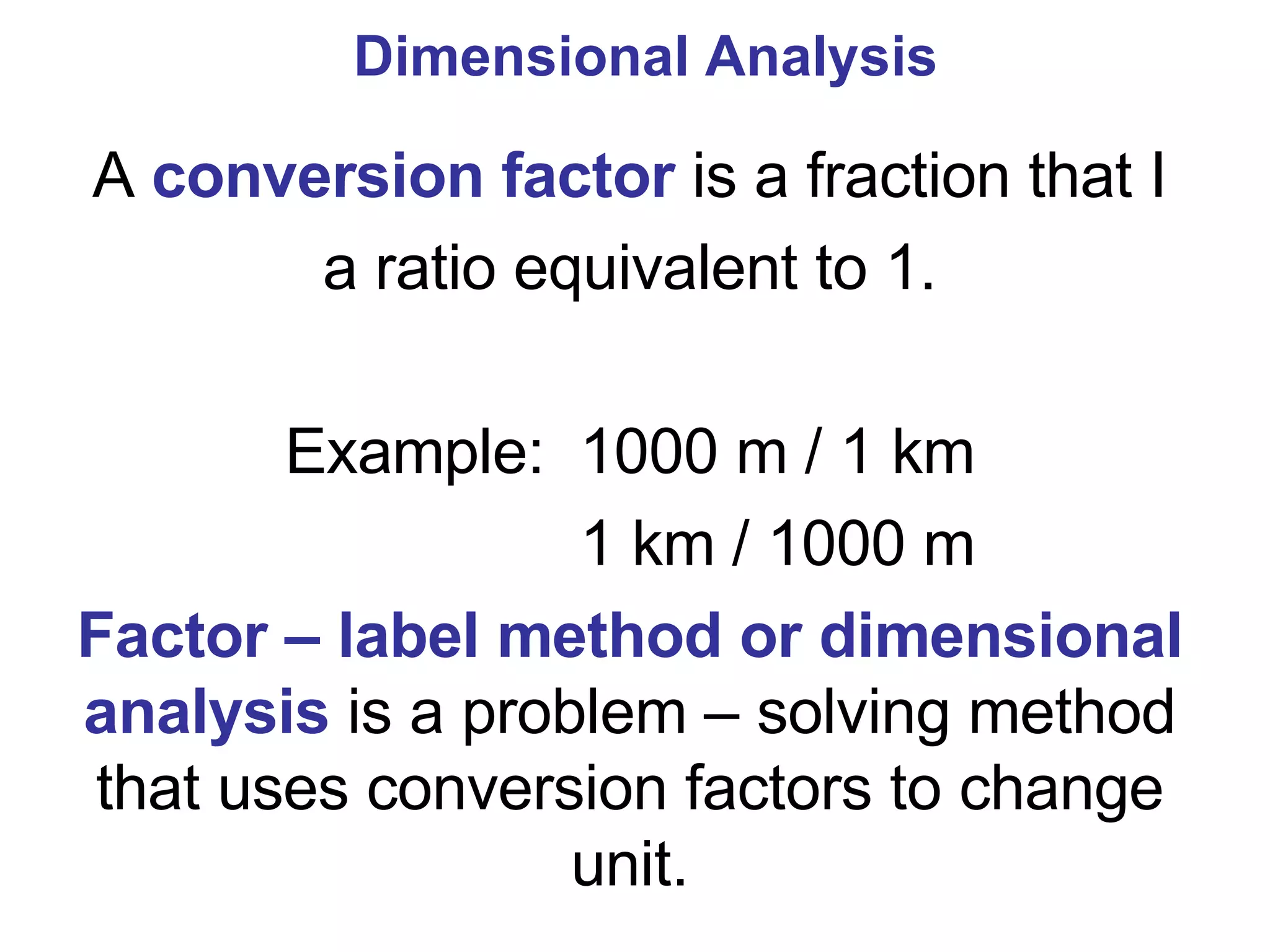
2. It explains the difference between elements, compounds, atoms, and molecules, and discusses measurement units and dimensional analysis.
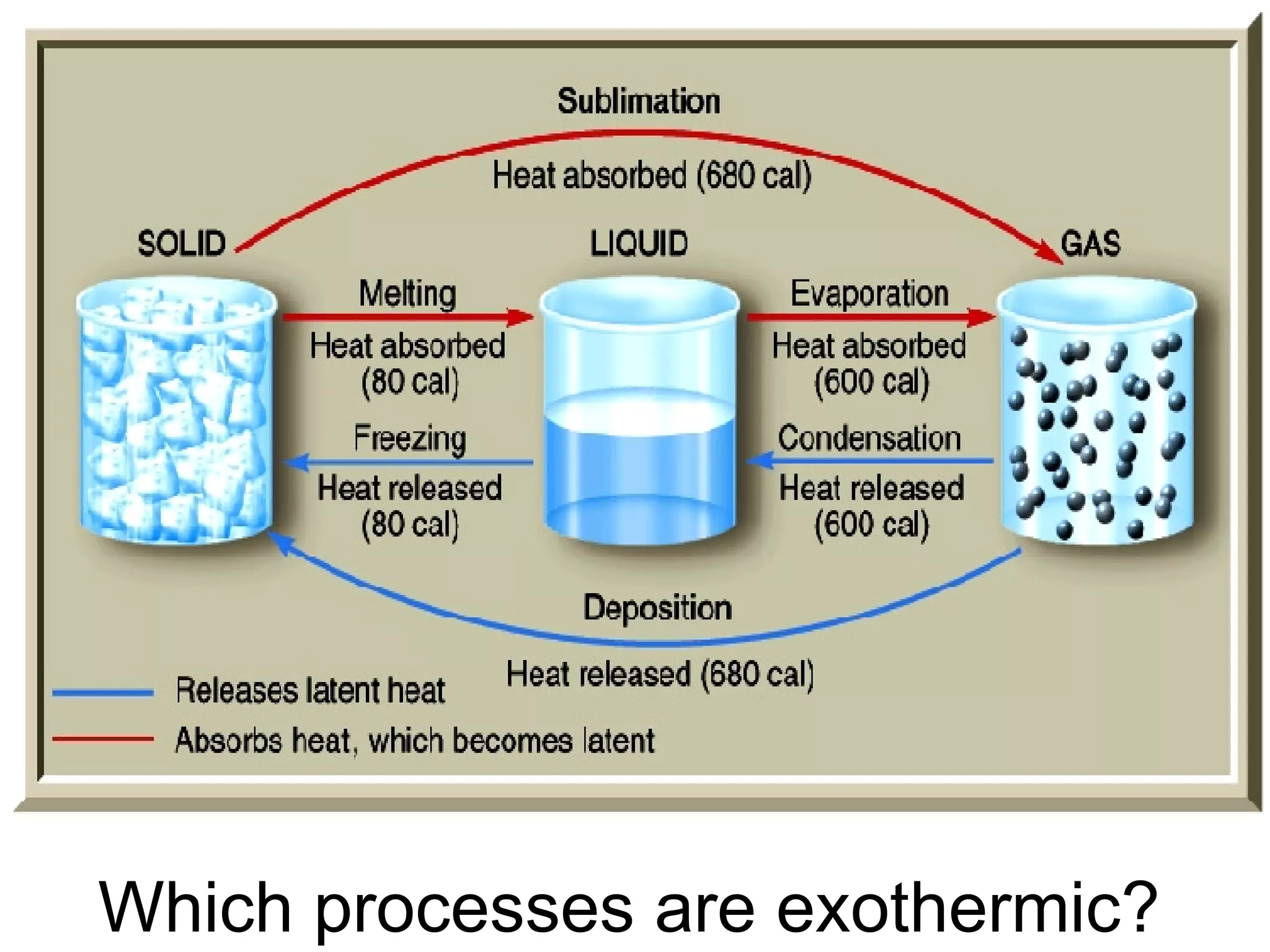
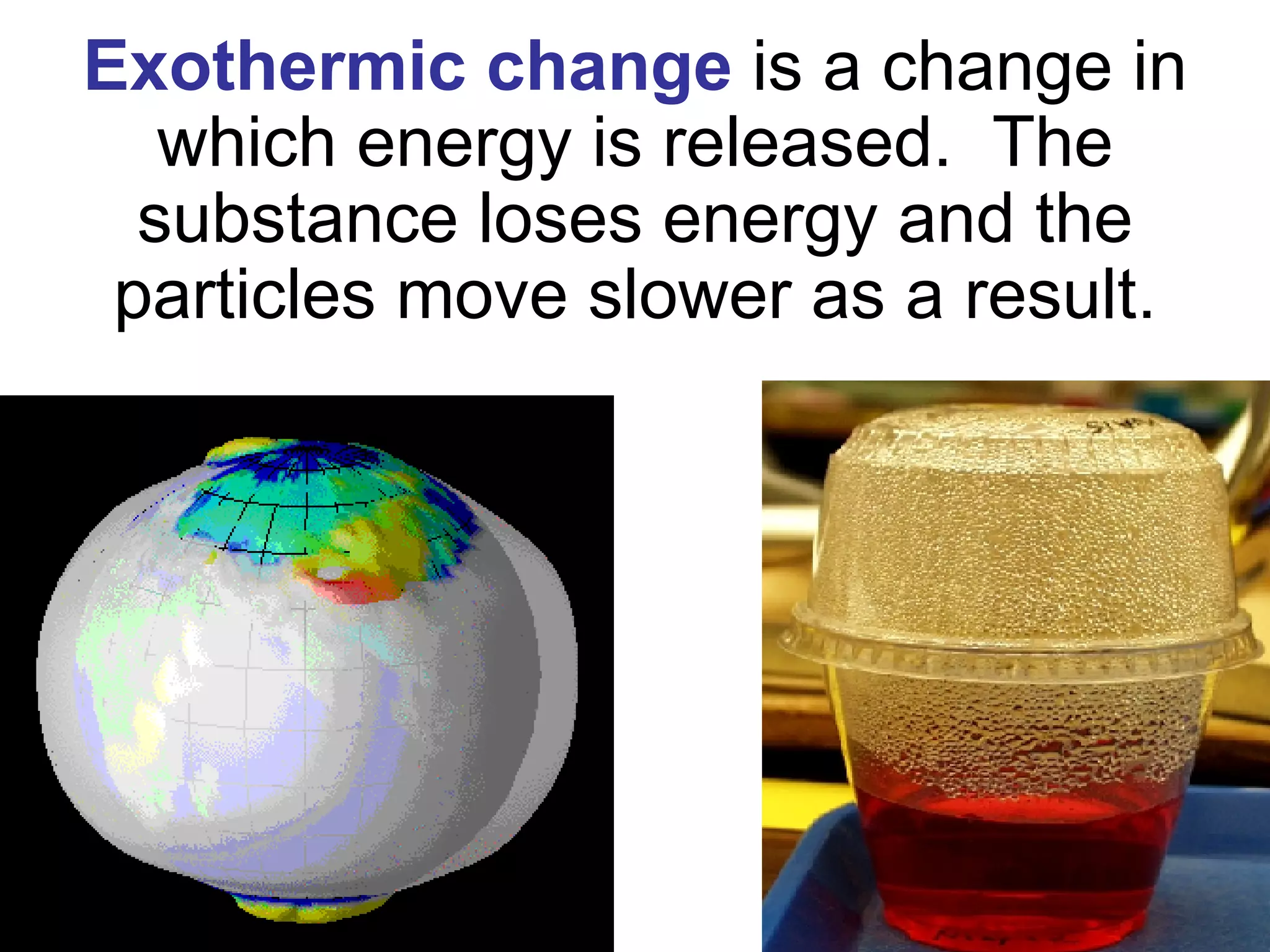
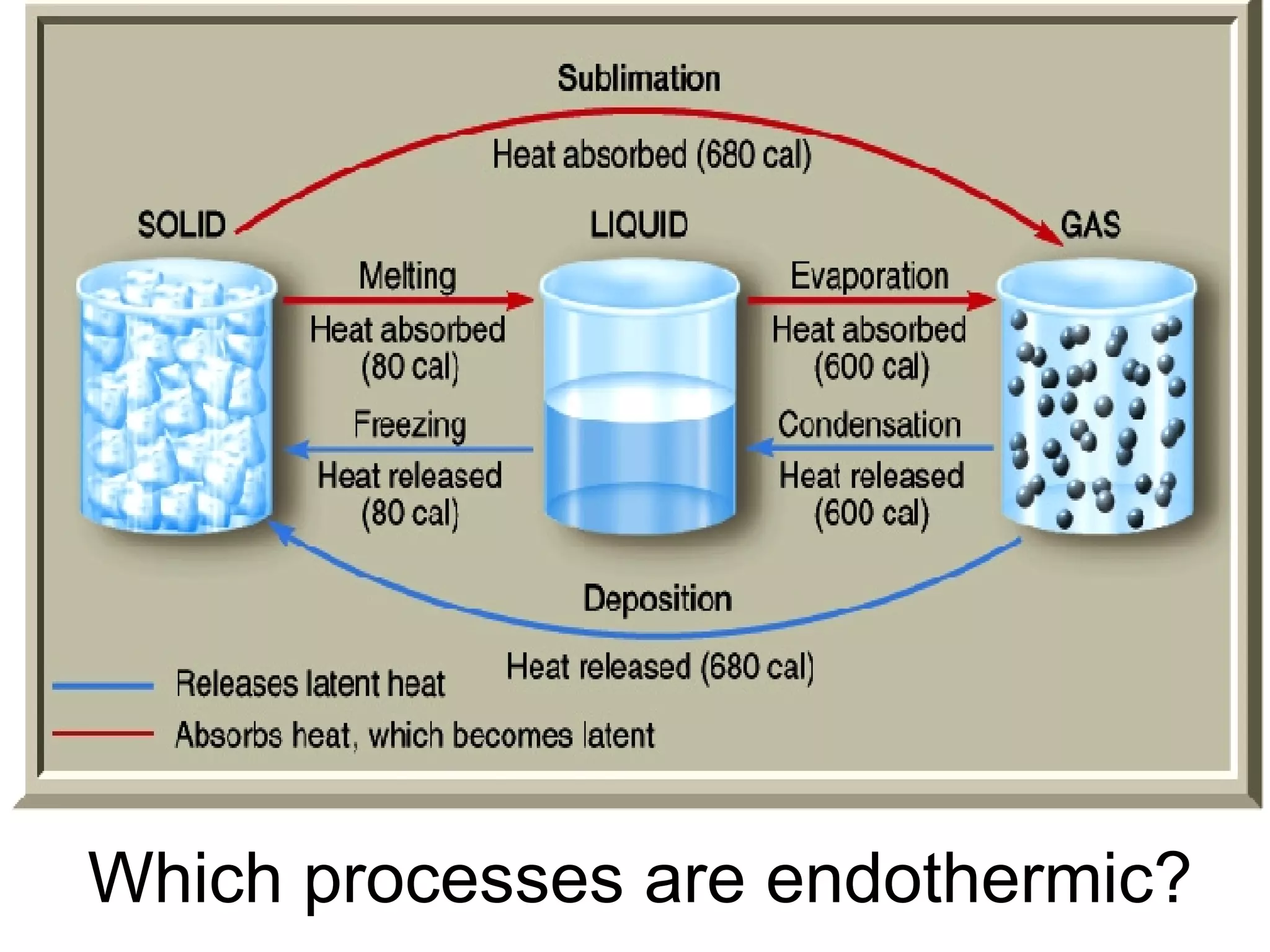
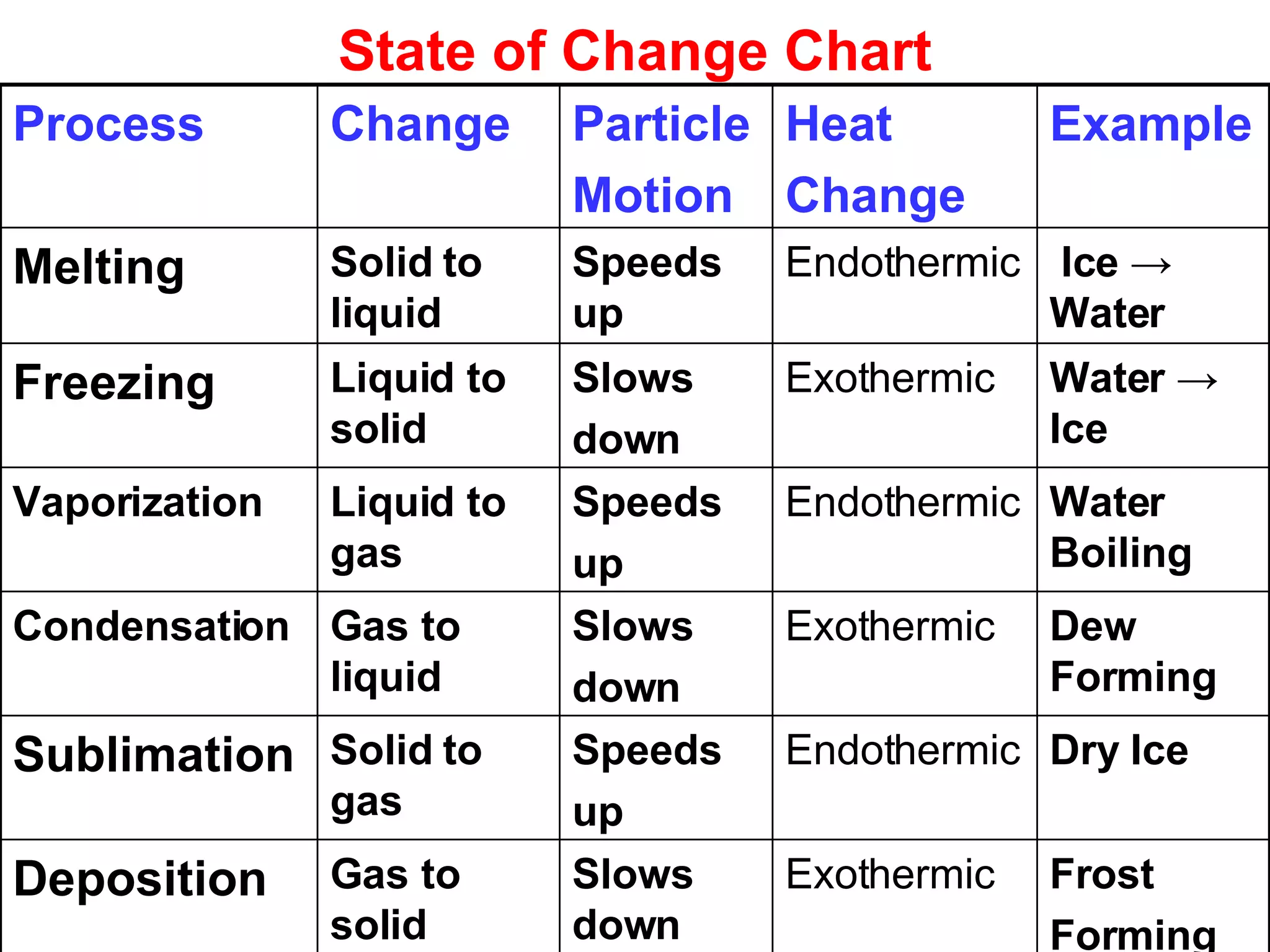
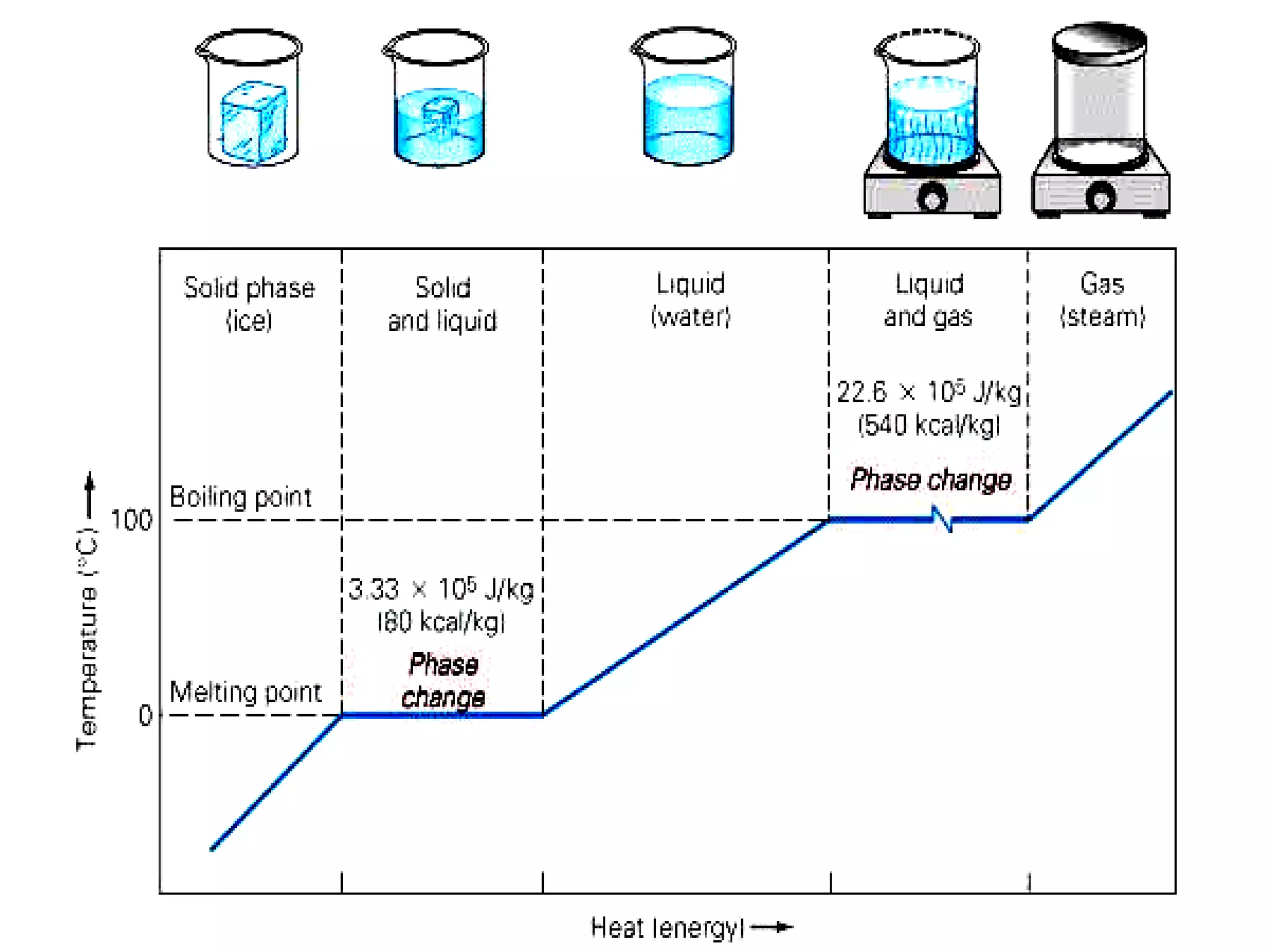
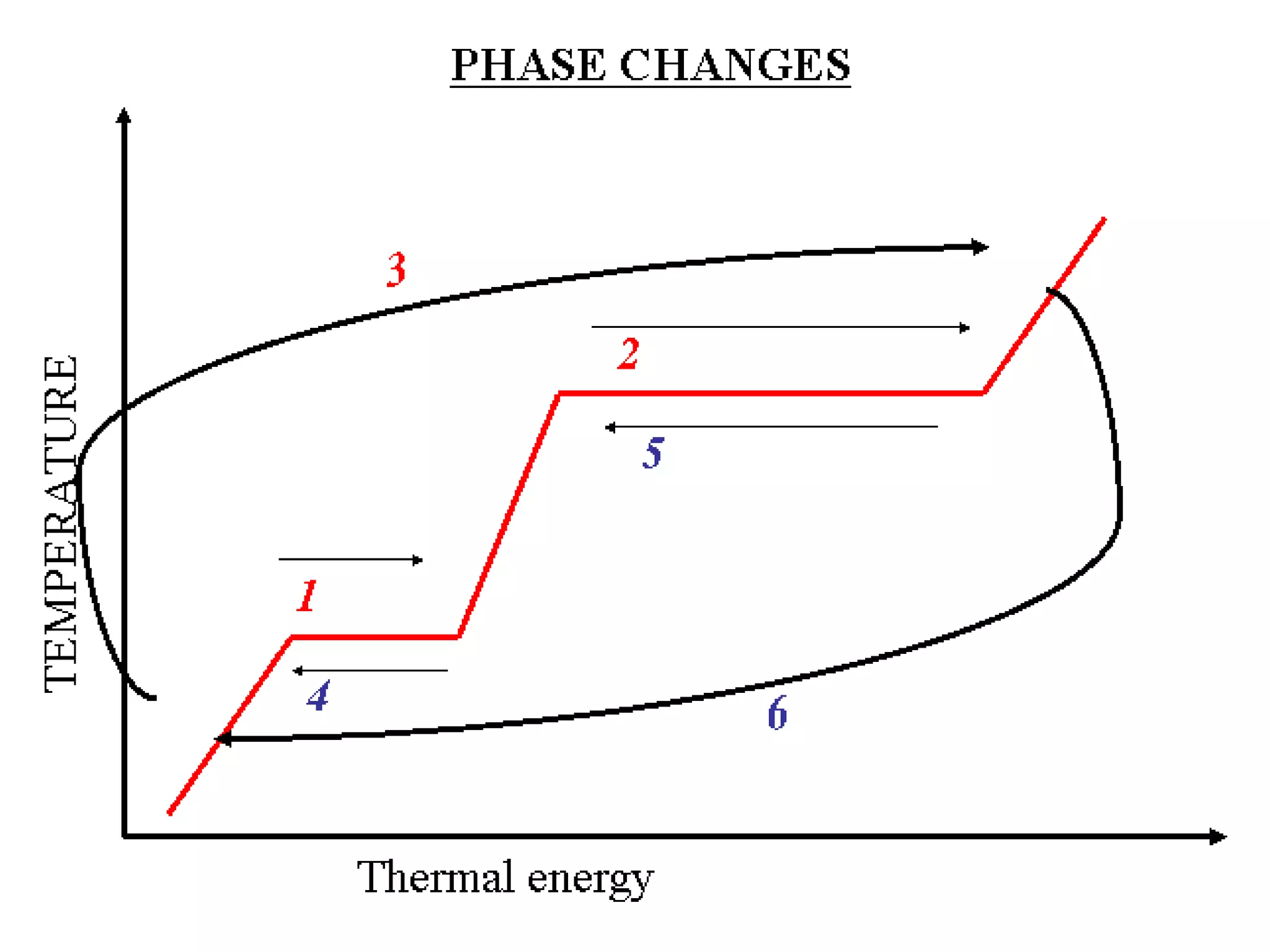
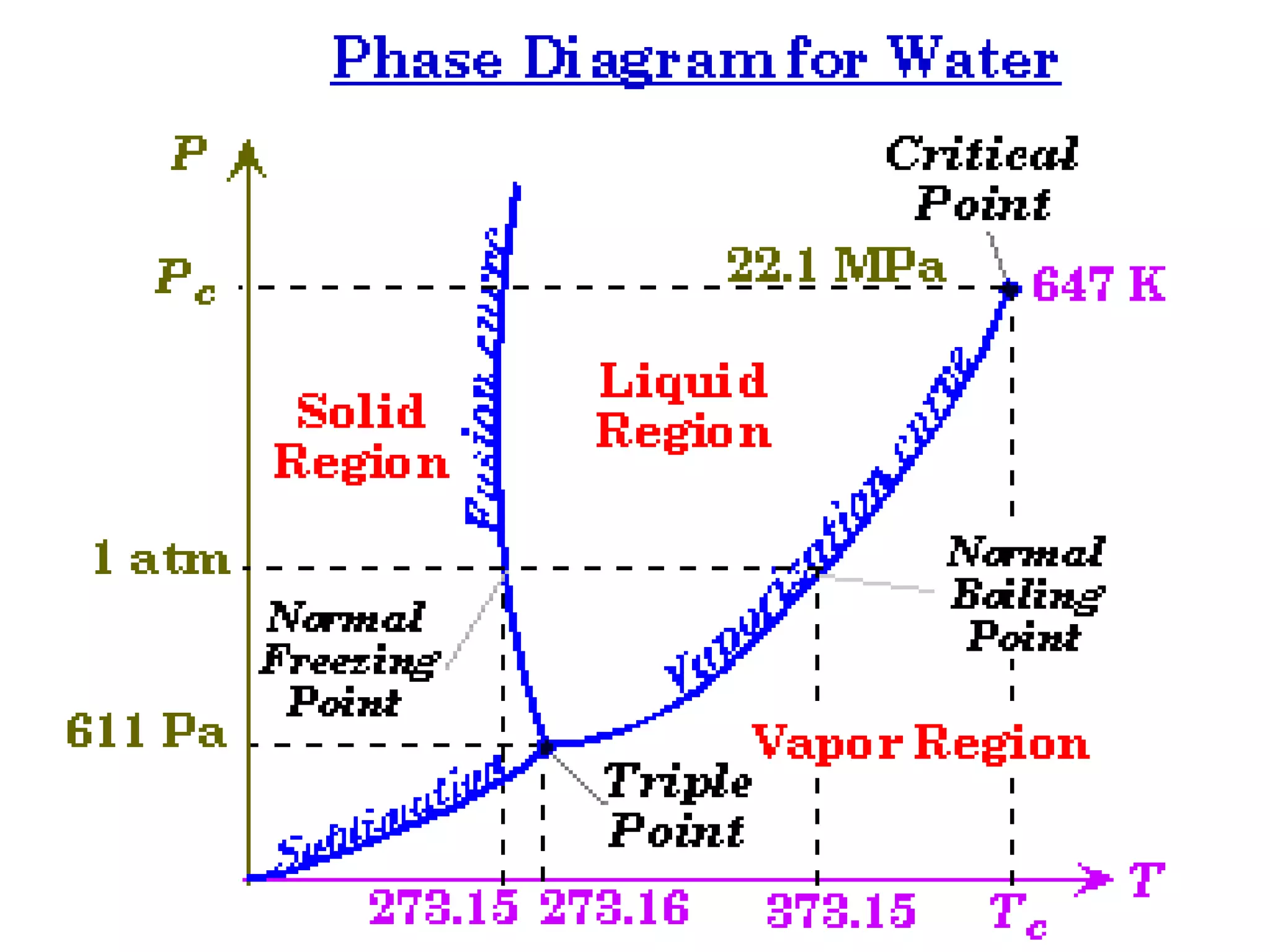
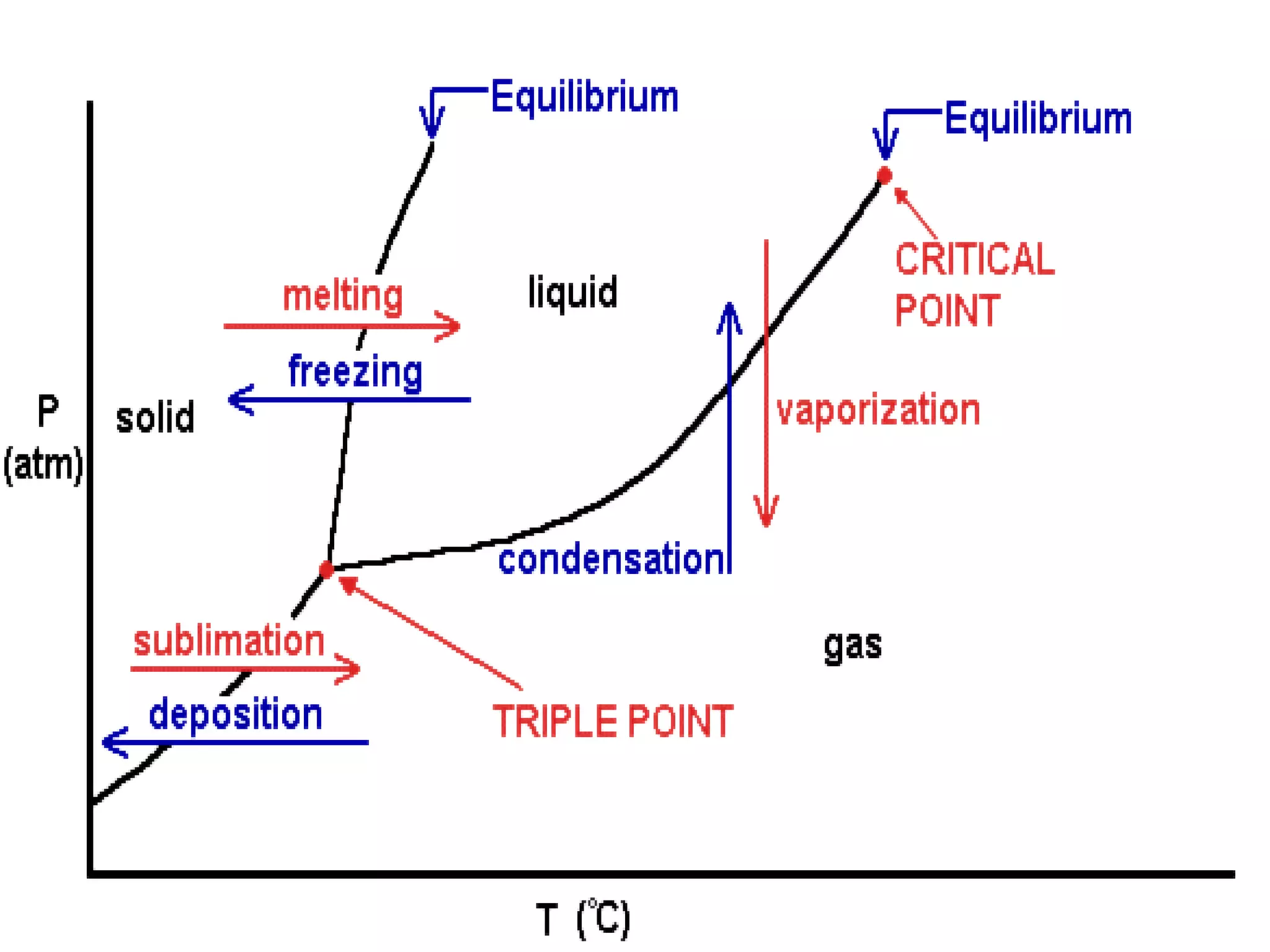
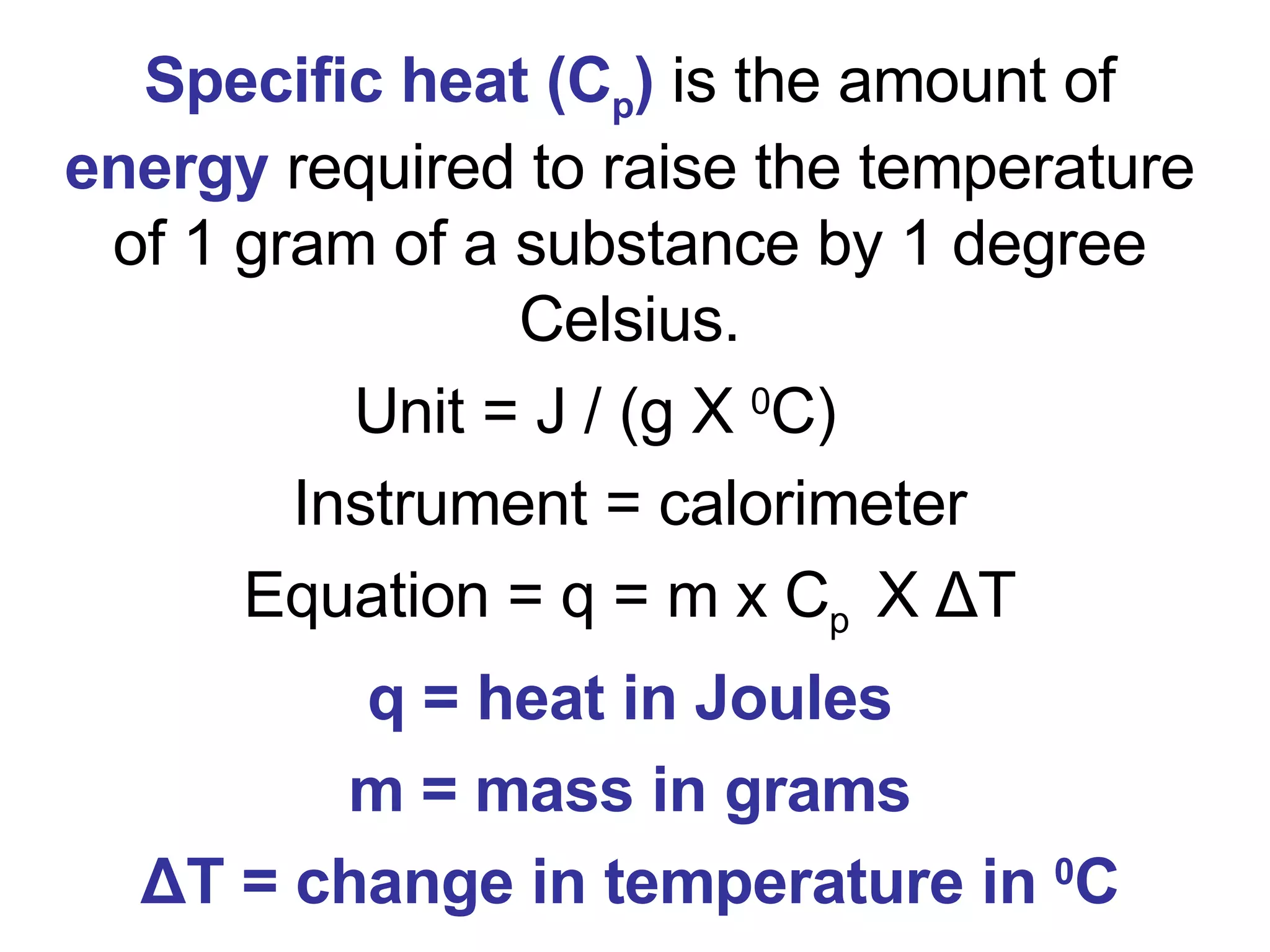
3. It covers concepts like specific heat, calorimetry, phase change diagrams, and Archimedes' principle for determining density.