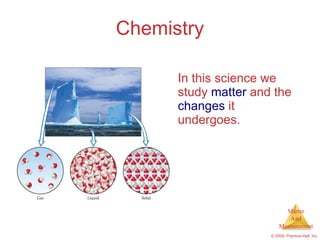
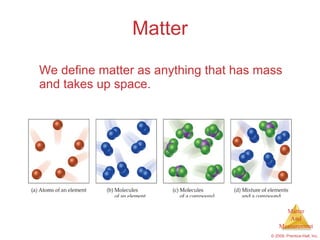
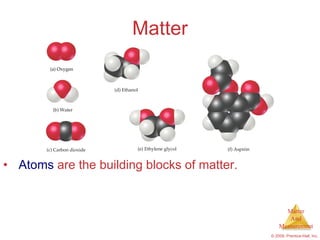
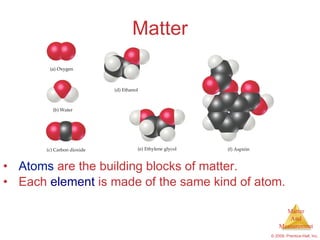
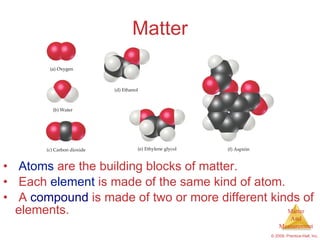
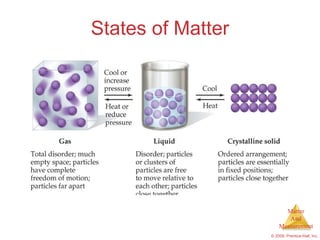
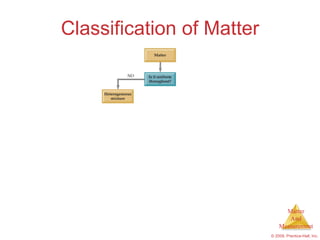
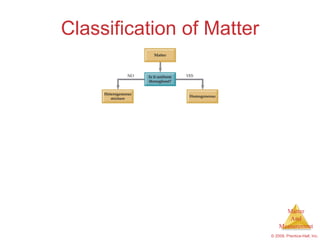
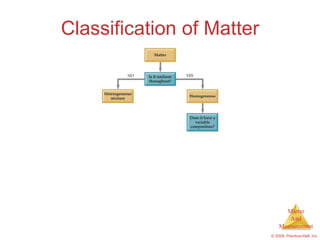
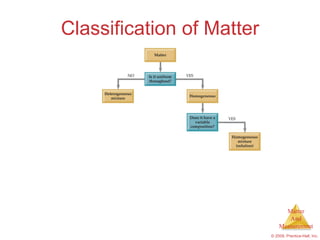
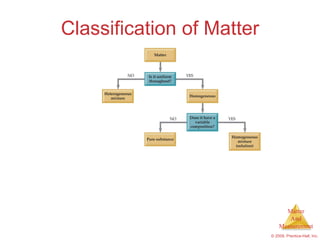
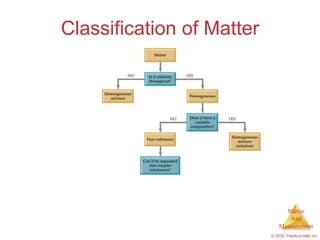
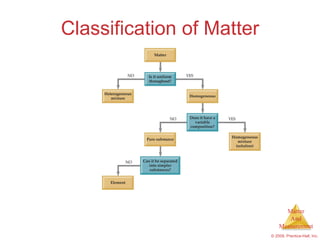
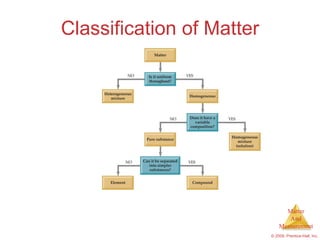
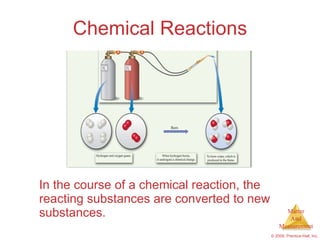
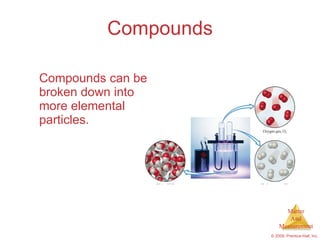
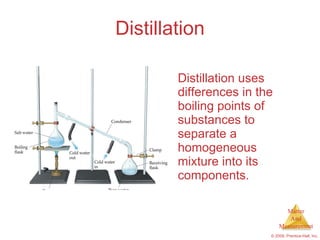
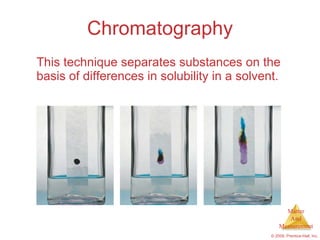
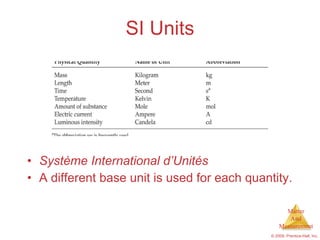
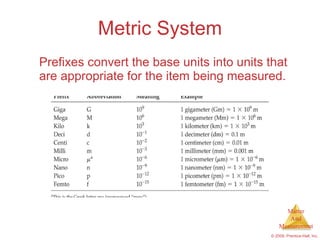
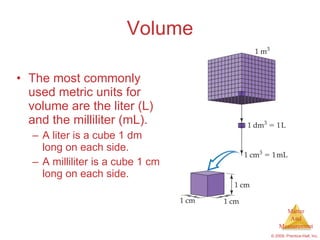
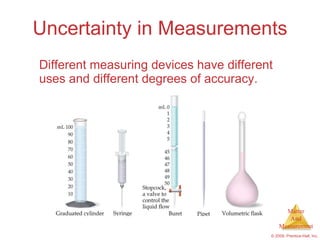
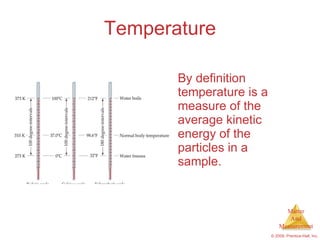
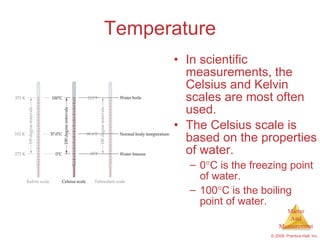
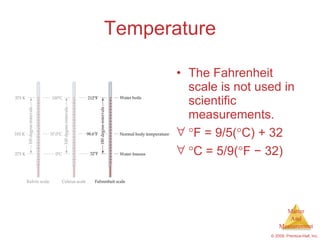
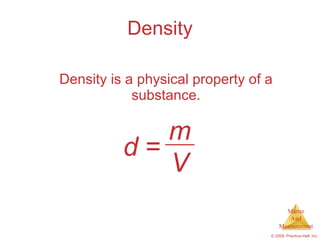
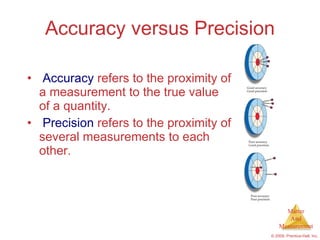
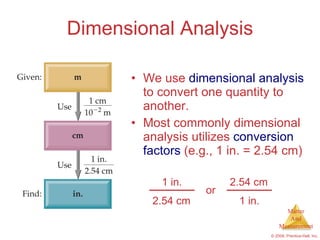
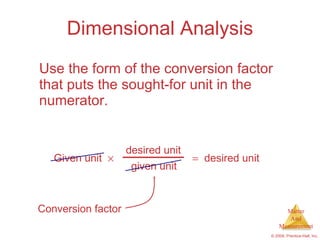
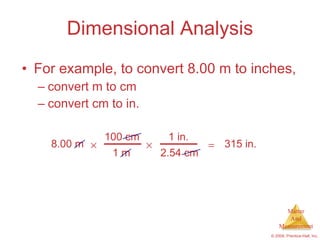
This chapter introduces the concepts of matter and measurement in chemistry. It defines matter as anything that has mass and takes up space, and states that atoms are the building blocks of matter. Atoms of the same element are all the same, while compounds are made of two or more different elements. The chapter also discusses the scientific method, physical and chemical properties and changes, classification of matter, units of measurement including the SI system, density, temperature scales, accuracy versus precision, and dimensional analysis.