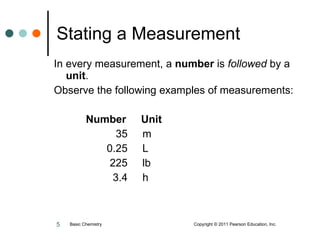
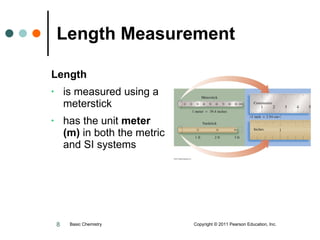
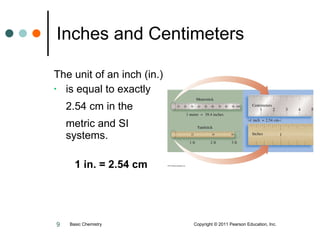
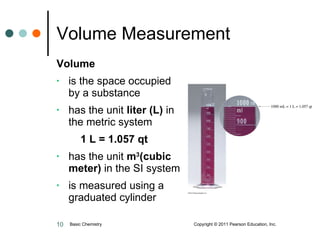
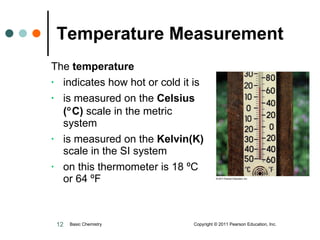
The document discusses units of measurement used in chemistry. It introduces common units like meters, liters, grams, seconds that are used to measure length, volume, mass and time. These units make up the International System of Units (SI) and metric system which are decimal systems used widely in science. Examples are given of measuring tools and conversions between units like inches to centimeters. Key types of measurements covered are length, volume, mass, temperature and time.