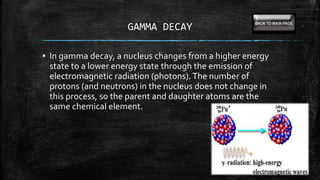
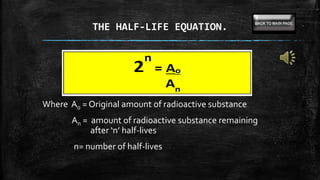
This document provides information on radioactive decay and half-life calculations. It defines key terms like radioactive decay, half-life, alpha decay, beta decay, gamma decay, and transmutation. It also describes how to use the half-life equation and time elapse equation to solve problems involving radioactive decay. Examples are provided to demonstrate solving for time elapsed given half-life and initial amount, and using carbon dating to determine the age of materials.