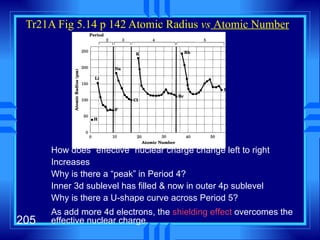
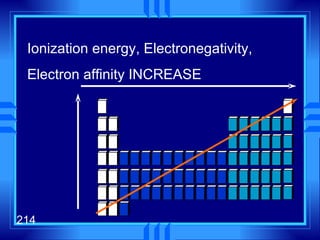
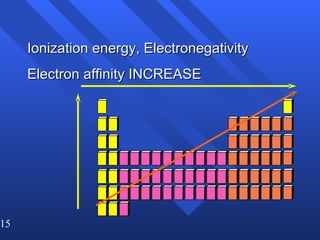
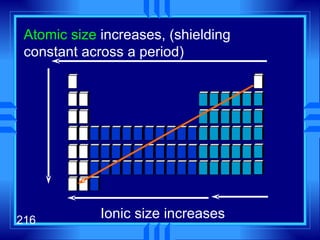
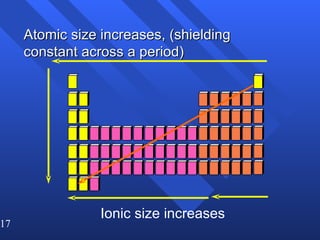
This document summarizes key concepts from a chapter on atomic structure:
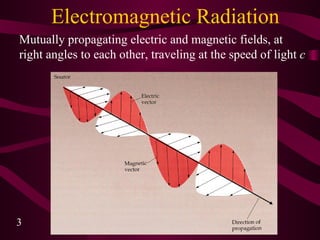
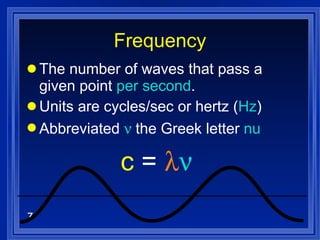
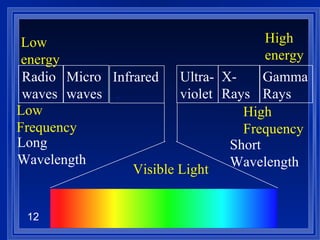
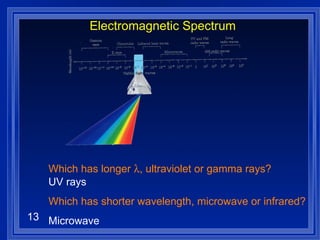
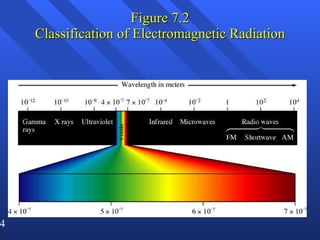
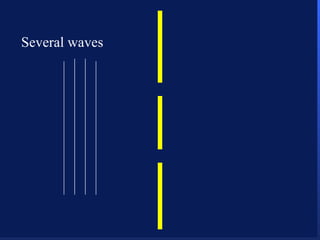
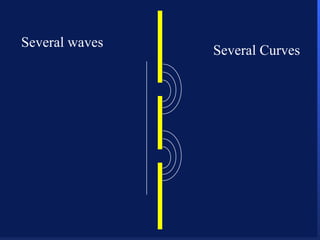
1. Electromagnetic radiation consists of oscillating electric and magnetic fields that propagate as waves. Light is a type of electromagnetic radiation detectable by the human eye.
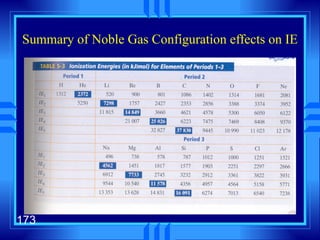
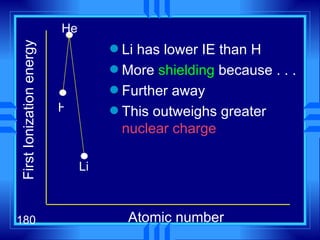
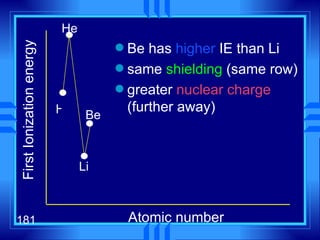
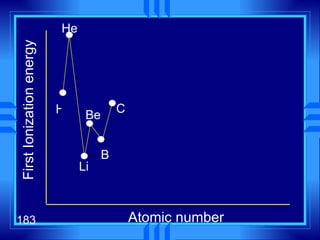
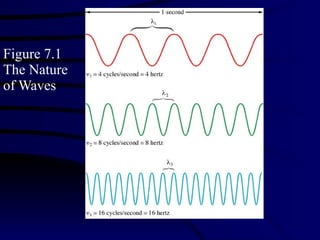
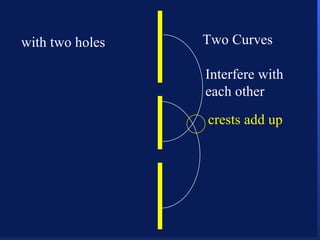
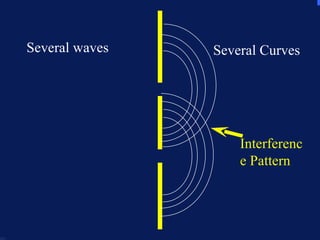
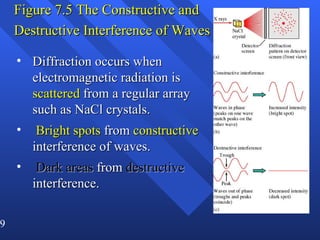
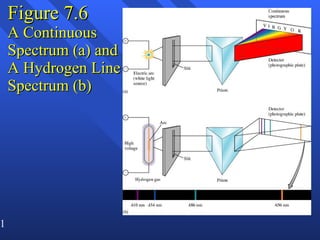
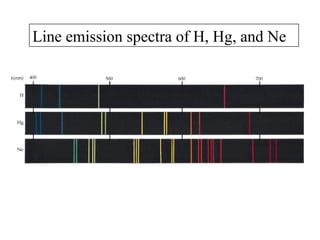
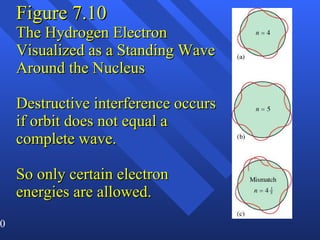
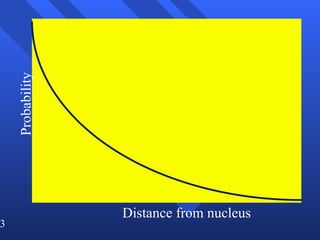
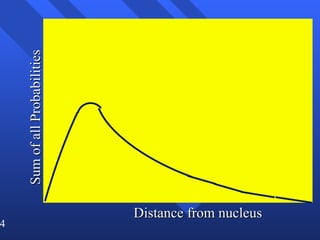
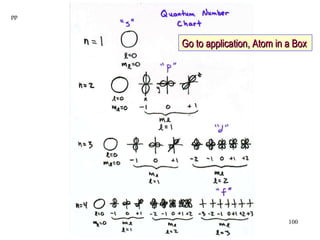
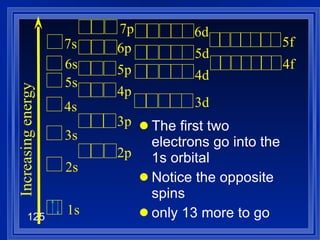
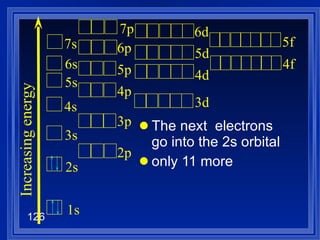
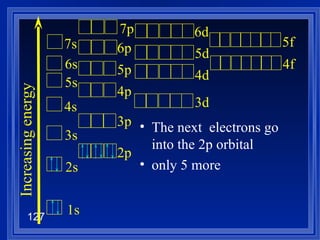
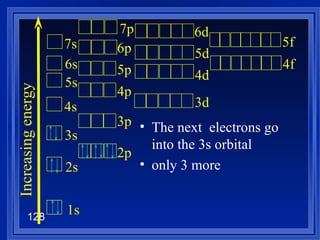
2. Atoms can only absorb or emit electromagnetic radiation at specific quantized energy levels. This explains the emission spectrum of hydrogen, which shows only certain discrete wavelengths.
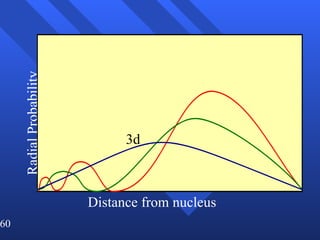
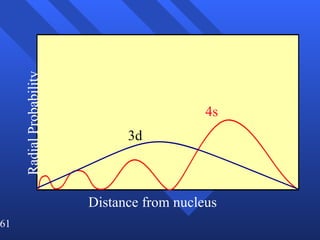
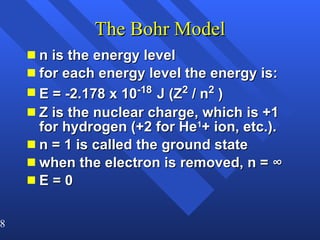
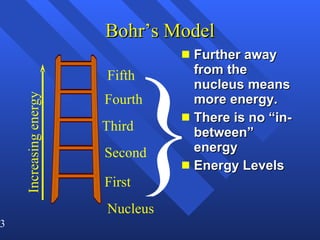
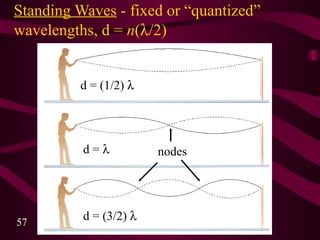
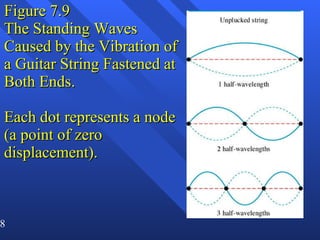
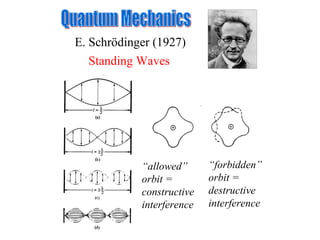
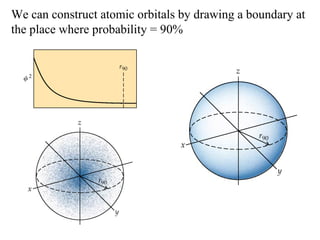
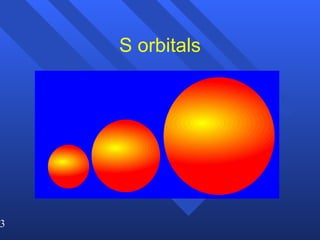
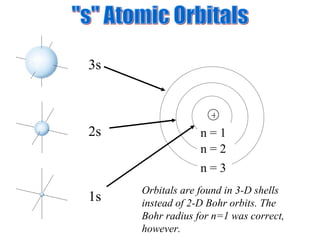
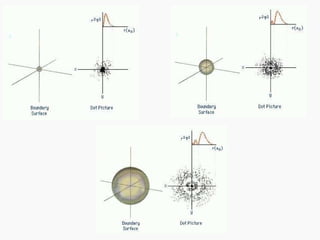
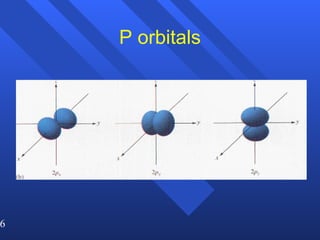
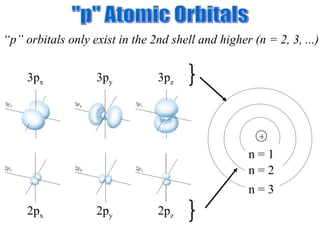
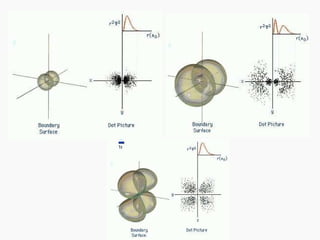
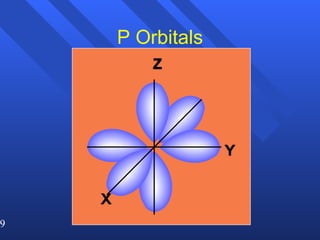
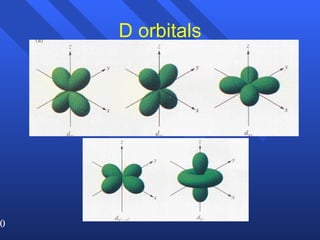
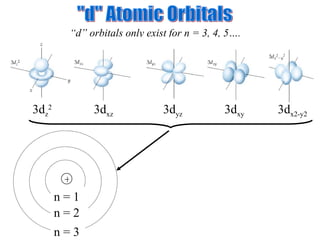
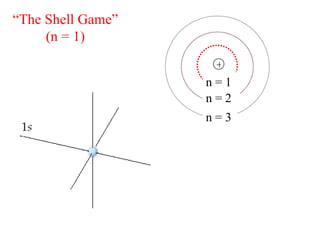
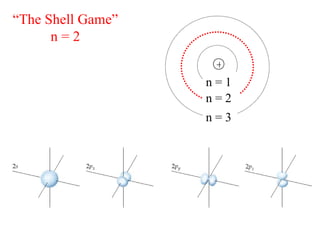
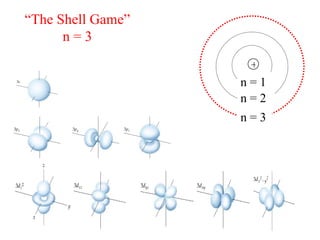
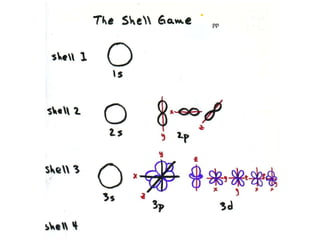
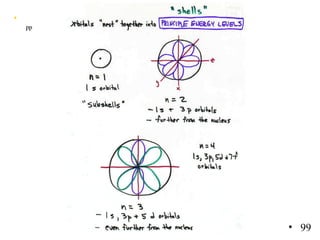
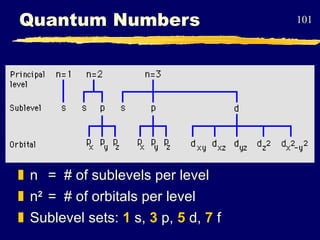
3. Niels Bohr's early model of the hydrogen atom proposed that electrons orbit the nucleus at fixed distances corresponding to discrete energy levels. Later, the quantum mechanical model described electron behavior as wave-like standing waves.
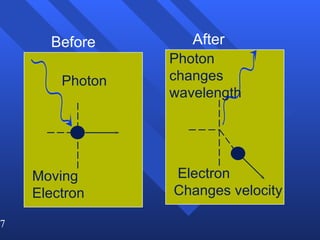
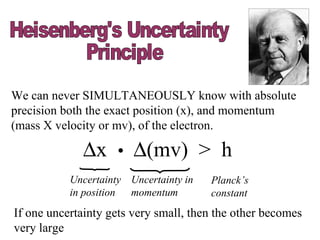
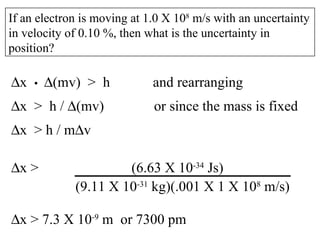
4. Werner Heisenberg's uncertainty principle states that the more precisely the position
















































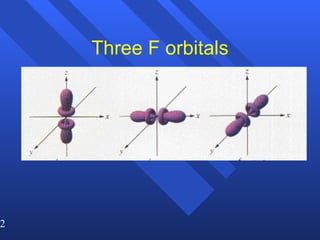
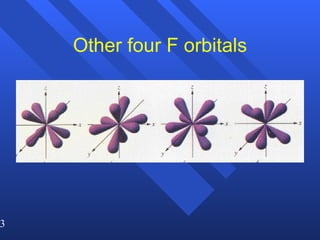
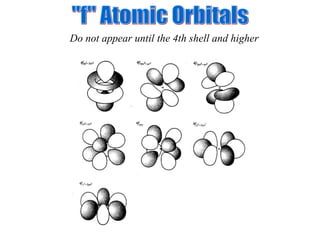









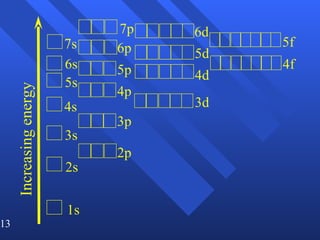








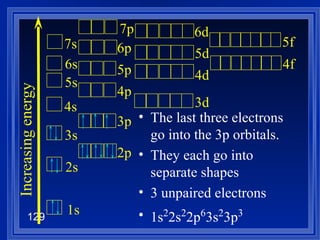







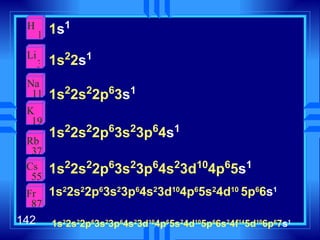

![Exceptions Ti = [Ar] 4s 2 3d 2 V = [Ar] 4s 2 3d 3 Cr = [Ar] 4s 1 3d 5 Mn = [Ar] 4s 2 3d 5 Half filled orbitals (only with d-orbitals). Scientists aren’t sure of why it happens. same for Cu [Ar] 4s 1 3d 10](https://image.slidesharecdn.com/ch7z5eatstructure-110115225106-phpapp02/85/Ch7-z5e-at-structure-144-320.jpg)

![More exceptions Lanthanum La: [Xe] 6s 2 5d 1 Cerium Ce: [Xe] 6s 2 4f 1 5d 1 Promethium Pr: [Xe] 6s 2 4f 3 5d 0 Gadolinium Gd: [Xe] 6s 2 4f 7 5d 1 Lutetium Pr: [Xe] 6s 2 4f 14 5d 1 Then we go back to Aufbau filling: Hafnium Hf: [Xe] 6s 2 4f 14 5d 2 We’ll just pretend that all except Cu and Cr follow the rules.](https://image.slidesharecdn.com/ch7z5eatstructure-110115225106-phpapp02/85/Ch7-z5e-at-structure-146-320.jpg)