Embed presentation
Downloaded 10 times

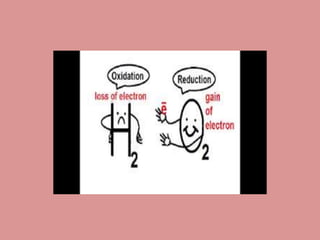

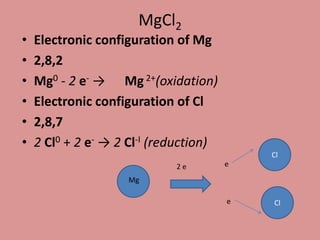

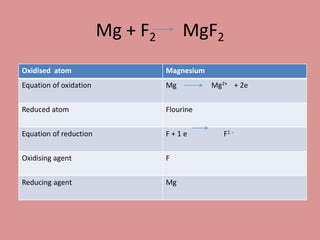

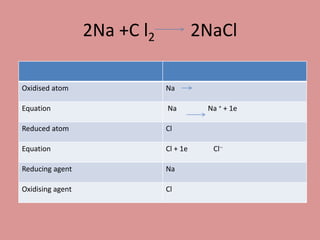
Oxidation is the loss of electrons, while reduction is the gain of electrons. The atom that loses electrons is the oxidizing agent, and the atom that gains electrons is the reducing agent. For example, in the reaction Mg + Cl2 → MgCl2, magnesium (Mg) is oxidized as it loses electrons and becomes Mg2+, while chlorine (Cl) is reduced as it gains electrons and becomes Cl-. Magnesium serves as the reducing agent and chlorine serves as the oxidizing agent.







