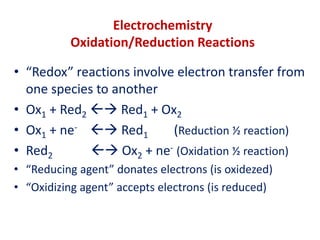
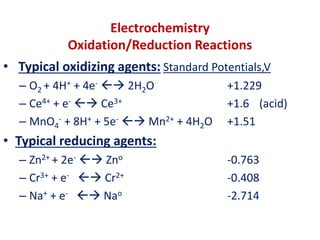
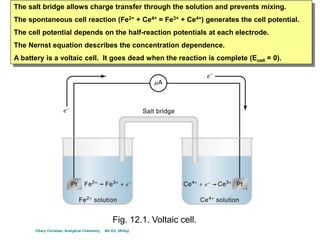
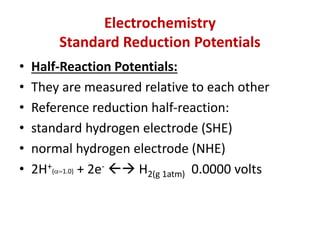
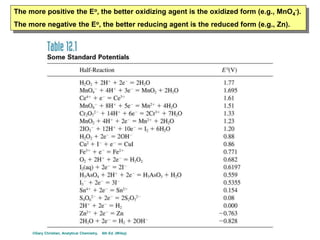
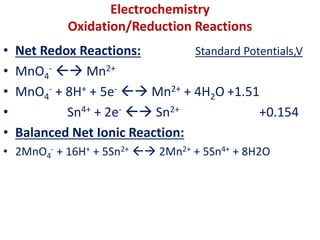
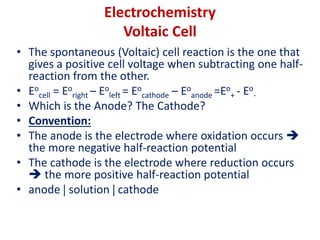
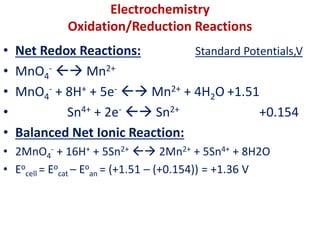
The document covers the fundamentals of electrochemistry, focusing on oxidation/reduction (redox) reactions and electrode potentials. It discusses standard reduction potentials for various oxidizing and reducing agents, illustrating their roles in voltaic cells and the computation of cell potential. Additionally, the Nernst equation is introduced to explain the effect of concentrations on electrode potentials.



![Electrochemistry
Nernst Equation
• Effects of Concentrations on Potentials:
• aOx + ne- bRed
• E = Eo – (2.3026RT/nF) log([Red]b/[Ox]a
– Where E is the reduction at specific conc.,
– Eo is standard reduction potential, n is number of electrons
involved in the half reaction,
– R is the gas constant (8.3143 V coul deg-1mol-1),
– T is absolute temperature,
– and F is the Faraday constant (96487 coul eq-1).
• At 25oC(298.16K) the value of 2.3026RT/F is 0.05916
• Note: Concentrations should be activities](https://image.slidesharecdn.com/electrochemistrypresentation-191113085835/85/Electrochemical-Cells-and-Electrode-Potentials-12-320.jpg)
![Electrochemistry
• Calculations:
• MnO4
- + 8H+ + 5e- Mn2+ + 4H2O Eo = +1.51 V
• For [H+] = 1.0M, [MnO4
-] = 0.10M, [Mn2+] = 0.010M
• E = Eo – 0.05916/5 (log ([Mn2+]/[MnO4
-][H+]8)
• E = +1.51 – 0.1183(-1) = +1.63 V vs NHE
• Note: This is more positive than Eo
• Greater tendency to be reduced compared to standard
conditions.](https://image.slidesharecdn.com/electrochemistrypresentation-191113085835/85/Electrochemical-Cells-and-Electrode-Potentials-13-320.jpg)
![Electrochemistry
• Calculations:
• Silver electrode/silver chloride deposit/0.010M NaCl
• AgCl + 1e- Ago + Cl- E = ?
• Ag+ + 1e- Ago Eo = +0.799 V
• AgCl Ag+ + Cl- Ksp= 1.8 x 10-10
• AgCl + e- Ago + Cl-
• E = Eo - (0.05916/1) Log (1/[Ag+])
• [Ag+] = Ksp/[Cl-] = 1.8 x 10-10/(0.010) = 1.8 x 10-8
• E = +0.799 – (0.05916)(7.74) = +0.341 V](https://image.slidesharecdn.com/electrochemistrypresentation-191113085835/85/Electrochemical-Cells-and-Electrode-Potentials-14-320.jpg)
