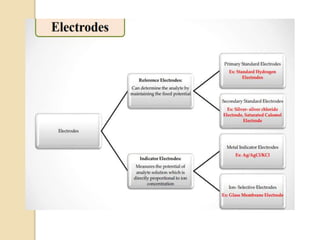
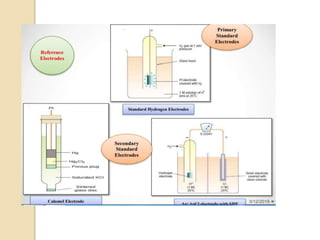
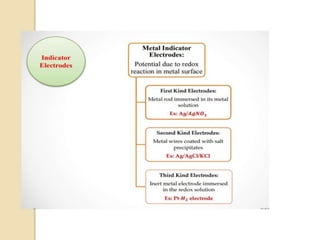
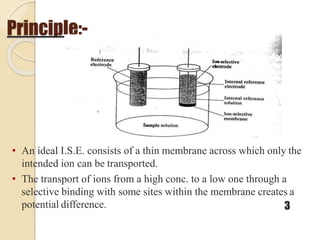
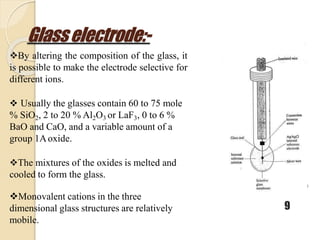
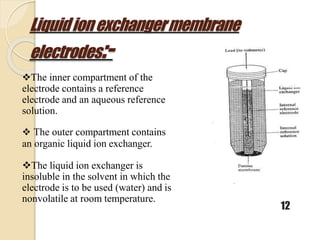
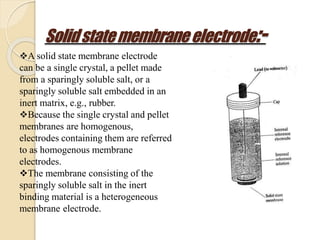
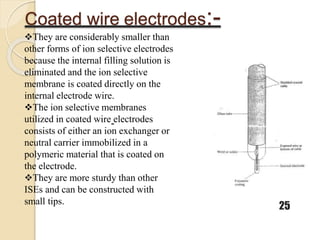
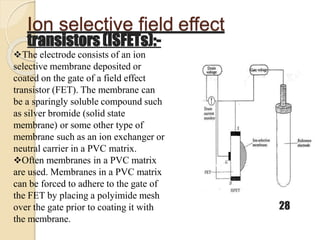
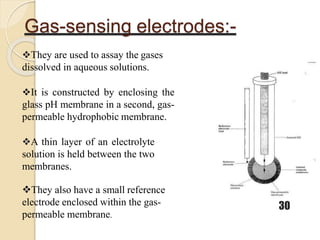
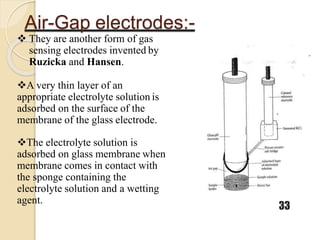
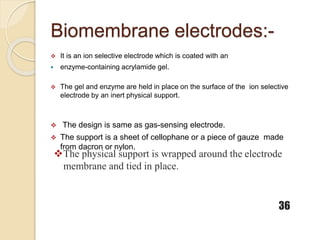
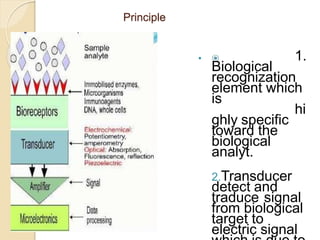
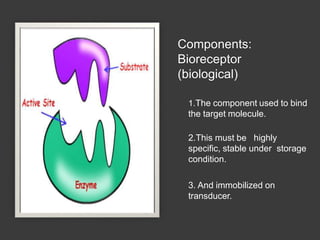
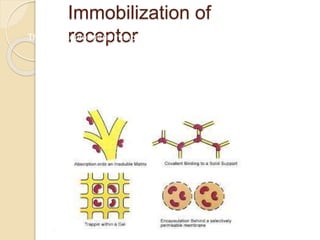
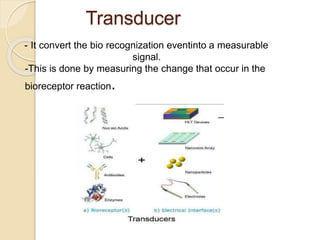
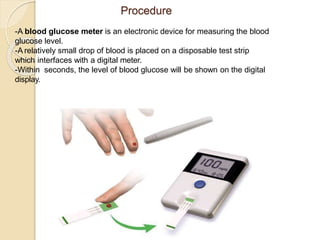
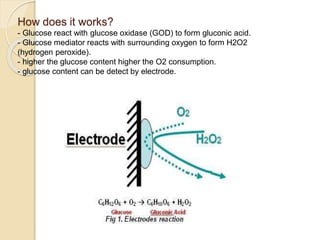
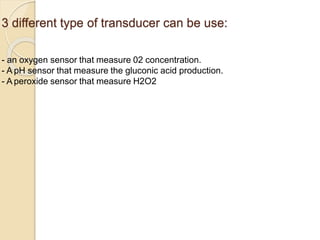
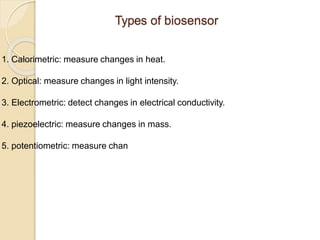
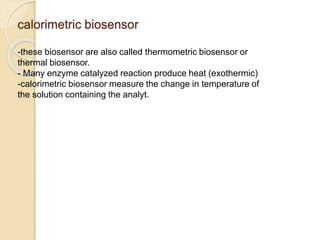
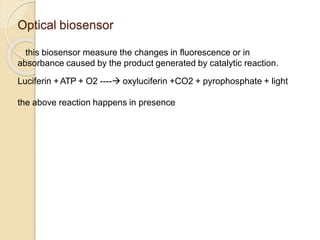
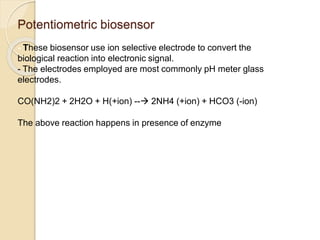
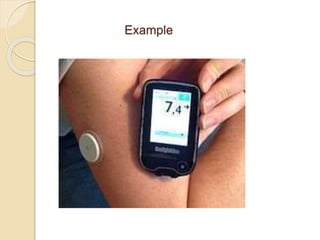
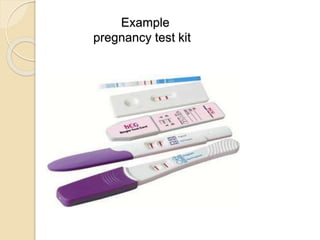
This document discusses different types of electrodes used in electroanalytical chemistry. It describes inert electrodes like platinum, gold and graphite that do not participate in reactions, and reactive electrodes like zinc, copper and lead that actively participate in reactions. The document discusses various types of electrodes in detail, including glass electrodes, liquid ion exchanger membranes, solid state membranes, neutral carrier membranes, coated wire electrodes, and ion selective field effect transistors. It also outlines the principle, advantages, limitations and applications of ion selective electrodes.